Complete the molecular orbital (MO) diagram of NH3. Assume the ground state electron configuration.
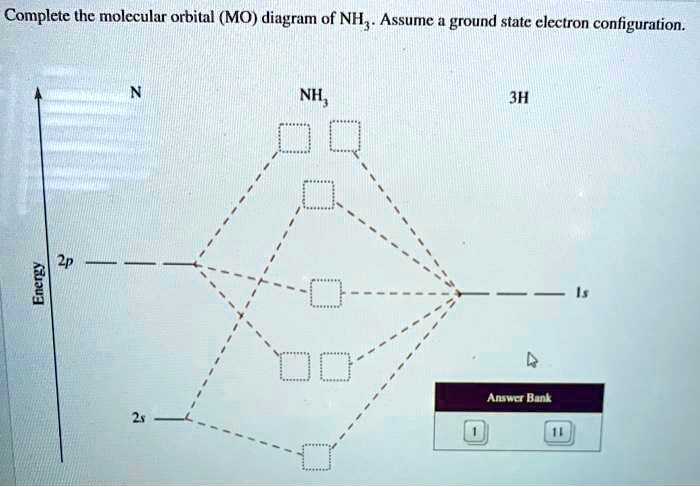
The Correct Answer and Explanation is:
Here is the completed molecular orbital (MO) diagram for NH₃, with the electrons filled in according to the ground state configuration.
Completed MO Diagram:
Here is a description of how the electrons are placed in the orbitals:
- Nitrogen (N) Atomic Orbitals (Left Side):
- 2s orbital: Contains a pair of electrons (↑↓).
- 2p orbitals: Contain three unpaired electrons (↑ ↑ ↑).
- Three Hydrogen (3H) Atomic Orbitals (Right Side):
- 1s orbitals: Contain a total of three unpaired electrons (↑ ↑ ↑), one from each hydrogen atom.
- Ammonia (NH₃) Molecular Orbitals (Center):
- Lowest bonding MO (1st box from the bottom): Contains a pair of electrons (↑↓).
- Degenerate bonding MOs (2nd and 3rd boxes from the bottom): Each contains a pair of electrons (↑↓).
- Non-bonding MO (4th box from the bottom): Contains a pair of electrons (↑↓). This is the Highest Occupied Molecular Orbital (HOMO) and represents the lone pair on the nitrogen.
- Degenerate antibonding MOs (top two boxes): These orbitals are empty. They are the Lowest Unoccupied Molecular Orbitals (LUMOs).
Explanation
To correctly complete the molecular orbital diagram for ammonia (NH₃), we follow a systematic process based on its ground state electron configuration.
First, we must determine the total number of valence electrons in the molecule. The central nitrogen atom is in Group 15 of the periodic table, so it contributes five valence electrons (2s²2p³). Each of the three hydrogen atoms is in Group 1 and contributes one valence electron (1s¹). Therefore, the total number of valence electrons for the NH₃ molecule is 5 + (3 × 1) = 8 electrons.
Next, we place these electrons into the atomic orbitals shown on the sides of the diagram. For the nitrogen atom, two electrons fill the 2s orbital, and three electrons singly occupy the three 2p orbitals according to Hund’s rule. For the three hydrogen atoms, their three collective electrons are placed in the energy level representing their 1s orbitals.
Finally, we fill the molecular orbitals in the center of the diagram with the eight total valence electrons, following the Aufbau principle by filling the lowest energy orbitals first. The lowest energy MO is a bonding orbital and is filled with a pair of electrons. The next level contains two degenerate (equal energy) bonding orbitals, which are filled with a total of four electrons (two pairs). The remaining two electrons are placed as a pair into the next available orbital, which is a non-bonding MO. This non-bonding orbital corresponds to the lone pair of electrons on the nitrogen atom and is the Highest Occupied Molecular Orbital (HOMO). The highest energy orbitals on the diagram are antibonding and remain empty since all eight valence electrons have been placed. This final configuration, with six electrons in bonding orbitals and two in a non-bonding orbital, correctly describes the three N-H single bonds and the nitrogen lone pair in ammonia
