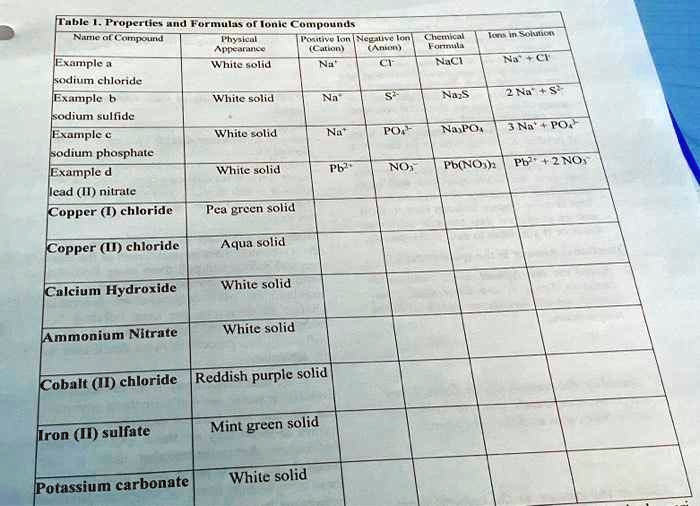
Properties and Formulas of Ionic
The Correct Answer and Explanation is:
Here is the completed table with the missing information filled in.
| Name of Compound | Physical Appearance | Positive Ion (Cation) | Negative Ion (Anion) | Chemical Formula | Ions in Solution |
| Example a<br>sodium chloride | White solid | Na⁺ | Cl⁻ | NaCl | Na⁺ + Cl⁻ |
| Example b<br>sodium sulfide | White solid | Na⁺ | S²⁻ | Na₂S | 2 Na⁺ + S²⁻ |
| Example c<br>sodium phosphate | White solid | Na⁺ | PO₄³⁻ | Na₃PO₄ | 3 Na⁺ + PO₄³⁻ |
| Example d<br>lead (II) nitrate | White solid | Pb²⁺ | NO₃⁻ | Pb(NO₃)₂ | Pb²⁺ + 2 NO₃⁻ |
| Copper (I) chloride | Pea green solid | Cu⁺ | Cl⁻ | CuCl | Cu⁺ + Cl⁻ |
| Copper (II) chloride | Aqua solid | Cu²⁺ | Cl⁻ | CuCl₂ | Cu²⁺ + 2 Cl⁻ |
| Calcium Hydroxide | White solid | Ca²⁺ | OH⁻ | Ca(OH)₂ | Ca²⁺ + 2 OH⁻ |
| Ammonium Nitrate | White solid | NH₄⁺ | NO₃⁻ | NH₄NO₃ | NH₄⁺ + NO₃⁻ |
| Cobalt (II) chloride | Reddish purple solid | Co²⁺ | Cl⁻ | CoCl₂ | Co²⁺ + 2 Cl⁻ |
| Iron (II) sulfate | Mint green solid | Fe²⁺ | SO₄²⁻ | FeSO₄ | Fe²⁺ + SO₄²⁻ |
| Potassium carbonate | White solid | K⁺ | CO₃²⁻ | K₂CO₃ | 2 K⁺ + CO₃²⁻ |
Explanation of a Correctly Completed Table
The provided table was completed by applying fundamental principles of inorganic chemistry, specifically the nomenclature and formulation of ionic compounds. The process for each empty row involves a logical sequence of steps to deduce the missing information.
First, the ions that form the compound must be identified from its name. For a compound like Copper (I) chloride, the name indicates the cation is copper with a +1 charge (Cu⁺), as specified by the Roman numeral (I). The anion is chloride, which is the ion of chlorine, always having a -1 charge (Cl⁻). For compounds with polyatomic ions, such as Calcium Hydroxide, one must know the formula and charge of the ions. Calcium (Ca) is in Group 2 of the periodic table, so it forms a Ca²⁺ ion, while hydroxide is a polyatomic ion with the formula OH⁻.
Second, the chemical formula is determined by balancing the charges of the cation and anion to achieve electrical neutrality. For Copper (I) chloride, the +1 charge of Cu⁺ and the -1 charge of Cl⁻ balance in a one to one ratio, giving the formula CuCl. For Calcium Hydroxide, one Ca²⁺ ion requires two OH⁻ ions to balance the charge. This results in the formula Ca(OH)₂, with parentheses used to indicate that the subscript 2 applies to the entire hydroxide ion.
Finally, the representation of ions in solution shows how the compound dissociates when dissolved. An ionic compound separates into its constituent ions. For example, Cobalt (II) chloride, with the formula CoCl₂, will dissociate into one cobalt ion (Co²⁺) and two chloride ions (2 Cl⁻). This process was systematically applied to each compound with missing data to accurately fill all the cells in the table.
