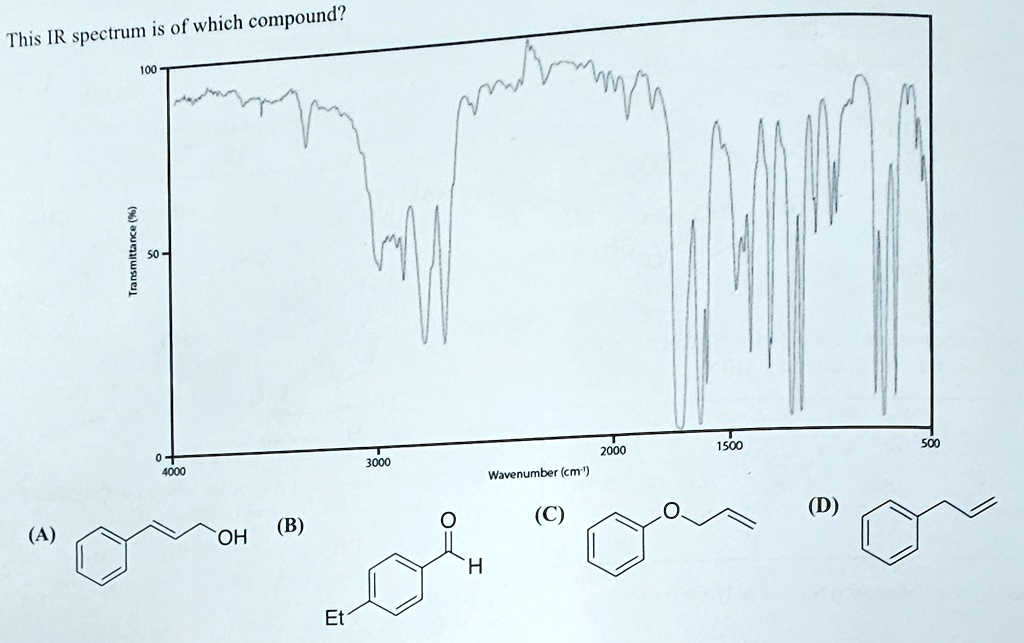
This IR spectrum is of which compound? 100 (A) Transmittance (%) 50 0 4000 (B) OH Et 3000 2000 1500 Wavenumber (cm
) (C) O H 500 (D)
The Correct Answer and Explanation is:
The correct answer is (A).
This IR spectrum corresponds to compound (A), cinnamyl alcohol, which is (E)-3-phenylprop-2-en-1-ol. Here is a step-by-step analysis of the spectrum to support this conclusion.
- O-H Stretch: The most prominent feature in the diagnostic region is the very broad, strong absorption band centered at approximately 3400 cm⁻¹. This is a classic signal for the O-H stretching vibration of an alcohol, broadened due to hydrogen bonding. This immediately suggests the presence of an -OH group, making compound (A) a strong candidate while ruling out compounds (B), (C), and (D), which do not contain an alcohol functional group.
- C-H Stretches: There are several peaks in the C-H stretching region. The sharp absorptions just above 3000 cm⁻¹ (around 3020-3080 cm⁻¹) are characteristic of C-H stretches from sp² hybridized carbons, found in both the benzene ring and the alkene. The weaker peaks just below 3000 cm⁻¹ are from the sp³ hybridized C-H bonds of the -CH₂- group.
- C=C Stretches: The spectrum shows a medium-intensity peak around 1650 cm⁻¹, which corresponds to the C=C double bond stretch of the alkene. Additionally, the sharp peaks in the 1600-1450 cm⁻¹ range are characteristic of C=C bond stretching within the aromatic (phenyl) ring.
- Fingerprint Region:
- C-O Stretch: A strong absorption is visible around 1050 cm⁻¹. This falls in the typical range (1000-1250 cm⁻¹) for a C-O single bond stretch, consistent with a primary alcohol like cinnamyl alcohol.
- Alkene C-H Bend: Crucially, there is a very strong, sharp peak at approximately 970 cm⁻¹. This is a highly characteristic out-of-plane C-H bending vibration for a trans-disubstituted alkene, confirming the stereochemistry shown for compound (A).
- Aromatic C-H Bends: The presence of a monosubstituted benzene ring is supported by strong absorption bands in the 770-730 cm⁻¹ and 690-710 cm⁻¹ range (out-of-plane C-H bending).
In contrast, the other options are inconsistent with the spectrum. Compound (B) is an aldehyde and would show a very strong C=O stretch around 1700 cm⁻¹ and characteristic aldehyde C-H stretches around 2720 and 2820 cm⁻¹, none of which are present. Compounds (C), an ether, and (D), a hydrocarbon, lack the alcohol -OH group necessary to explain the broad peak at 3400 cm⁻¹. Therefore, the combination of all observed peaks uniquely identifies the compound as cinnamyl alcohol
