Using the above IR spectra as a reference, determine the identity of the compound that would generate the following IR spectrum: (Hint: Only refer to the assigned peaks in the reference spectra for comparison) Unknown IR spectrum
The Correct Answer and Explanation is:
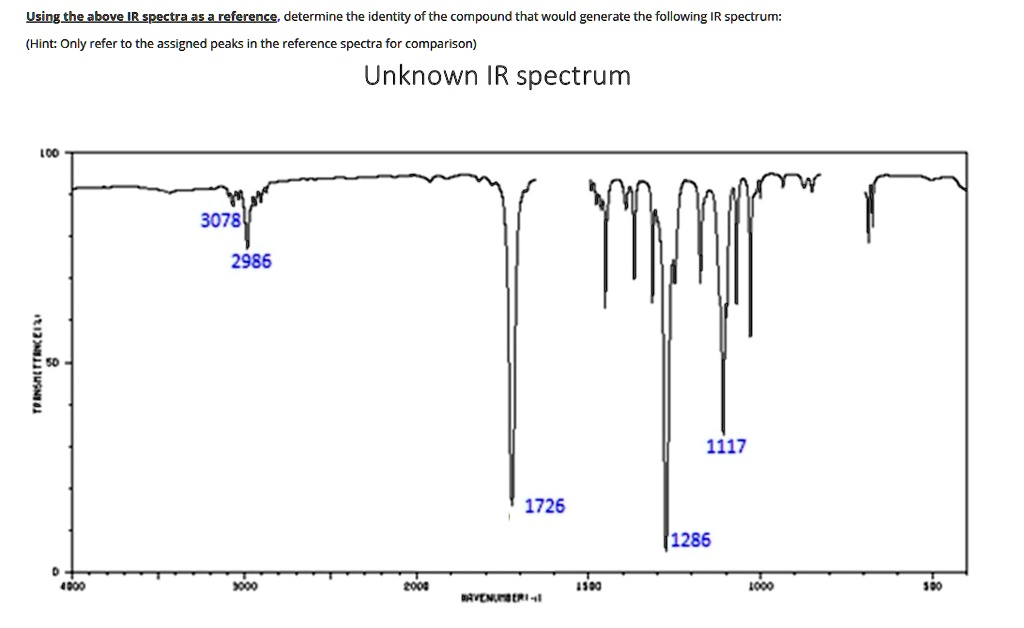
Based on the analysis of the provided infrared (IR) spectrum, the unknown compound is Methyl Acrylate.
The identification of this compound is based on a systematic interpretation of the key absorption peaks highlighted in the spectrum. Each peak corresponds to the vibration of specific chemical bonds within the molecule, allowing for the determination of the functional groups present.
First, the most prominent peak in the spectrum is the very strong, sharp absorption at 1726 cm⁻¹. This peak falls squarely in the carbonyl region (1650-1850 cm⁻¹) and is characteristic of a carbonyl (C=O) double bond stretch. The exact position is crucial; a value around 1726 cm⁻¹ is typical for an α,β unsaturated ester, where the carbonyl group is conjugated with a carbon carbon double bond.
This initial hypothesis of an ester is strongly supported by the two intense peaks in the fingerprint region at 1286 cm⁻¹ and 1117 cm⁻¹. This pair of absorptions is a classic signature for the C-O single bond stretching vibrations within an ester functional group. The higher frequency peak (1286 cm⁻¹) generally corresponds to the C(=O)-O stretch, while the lower one (1117 cm⁻¹) corresponds to the O-C stretch.
Next, we analyze the C-H stretching region, which is found between 2800 and 3300 cm⁻¹. The spectrum shows two distinct types of C-H bonds. The peak at 2986 cm⁻¹, which is just below the 3000 cm⁻¹ threshold, indicates the presence of C-H bonds on sp³ hybridized (alkane-like) carbon atoms. This confirms the molecule contains an alkyl component, such as a methyl or ethyl group.
In contrast, the peak at 3078 cm⁻¹, appearing just above the 3000 cm⁻¹ line, is characteristic of C-H bonds on sp² hybridized (alkene-like) carbon atoms. This signals the presence of a carbon carbon double bond (a vinyl group) in the structure.
Synthesizing all these pieces of evidence, the molecule must be an ester that contains both an alkyl group and a vinyl group, with the vinyl group conjugated to the carbonyl. The simplest structure that satisfies all these requirements is methyl acrylate (CH₂=CH-COOCH₃). This structure perfectly accounts for the sp² C-H stretches of the vinyl group, the sp³ C-H stretches of the methyl group, the conjugated ester C=O stretch, and the two C-O single bond stretches.
