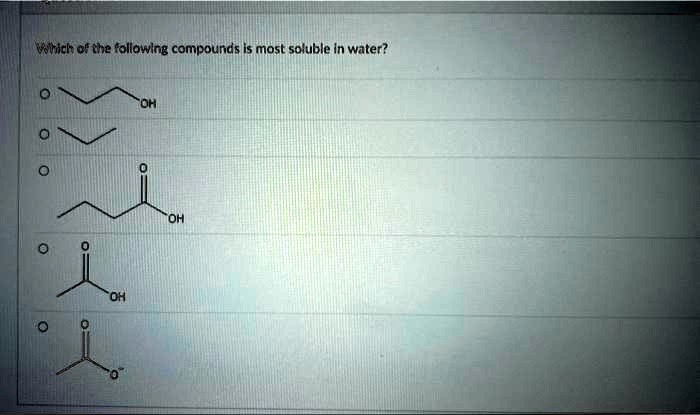
Which of the following compounds is most soluble in water?
The Correct Answer and Explanation is:
The correct answer is the fifth option, which is the propanoate ion.
The solubility of a compound in water is primarily governed by the principle of “like dissolves like.” Water is a highly polar solvent that can form extensive hydrogen bonds. Therefore, substances that are either ionic or polar tend to be the most soluble in water because they can form strong intermolecular interactions with water molecules.
Let’s analyze the given compounds:
- Propane (second option): This is a nonpolar alkane. It cannot form hydrogen bonds with water and can only interact through weak London dispersion forces. As a result, it is virtually insoluble in water.
- Propan-1-ol (first option): This alcohol contains a polar hydroxyl (-OH) group, which can participate in hydrogen bonding with water. The three-carbon chain is relatively short, so the polar nature of the -OH group makes the molecule readily soluble in water.
- Butanoic acid (third option) and Propanoic acid (fourth option): These are carboxylic acids. The carboxylic acid group (-COOH) is even more polar than a hydroxyl group and can form strong hydrogen bonds with water at two sites, the carbonyl oxygen and the hydroxyl group. This makes them very water-soluble. However, as the nonpolar carbon chain gets longer, solubility decreases. Therefore, propanoic acid (three carbons) is more soluble than butanoic acid (four carbons).
- Propanoate ion (fifth option): This molecule is the conjugate base of propanoic acid and is an ion, carrying a full negative charge. Ionic compounds are typically very soluble in water. The charged carboxylate group (-COO⁻) interacts with the partial positive charges on the hydrogen atoms of water molecules through extremely strong ion-dipole interactions. These ion-dipole forces are significantly stronger than the hydrogen bonds that neutral molecules like alcohols or carboxylic acids can form. The immense energetic favorability of these interactions makes the propanoate ion exceptionally soluble in water, far more so than its neutral counterparts.
