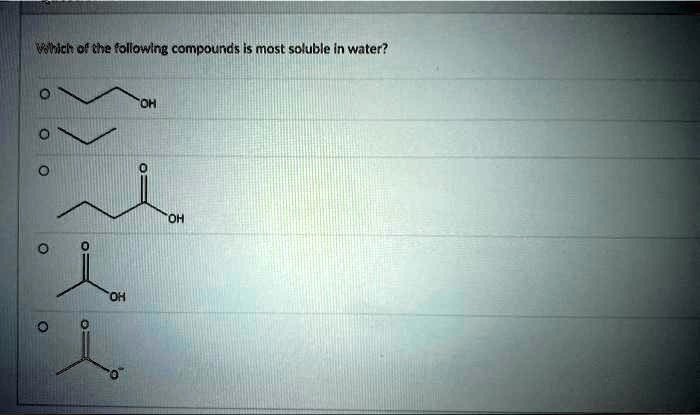
Which of the following compounds is most soluble in water?
The Correct Answer and Explanation is:
The correct answer is the last option, the propanoate ion.
The principle governing solubility is “like dissolves like.” Water is a highly polar solvent that can form extensive hydrogen bonds. Therefore, compounds that are either ionic or highly polar will be the most soluble in water. To determine which of the given compounds is most soluble, we must evaluate the strength of their interactions with water molecules.
The second compound, propane, is a nonpolar hydrocarbon. It cannot form hydrogen bonds or strong dipole-dipole interactions with water, so it will be virtually insoluble.
The first, third, and fourth compounds are propanol, butanoic acid, and propanoic acid, respectively. All three are neutral molecules containing polar functional groups (a hydroxyl group in the alcohol and a carboxyl group in the acids) that can participate in hydrogen bonding with water. This makes them all somewhat soluble. The solubility of these molecules is a balance between their polar, water-loving (hydrophilic) functional groups and their nonpolar, water-fearing (hydrophobic) carbon chains. As the nonpolar carbon chain gets longer, solubility in water decreases. Thus, propanoic acid is more soluble than butanoic acid.
The crucial comparison is between the neutral molecules and the fifth option, the propanoate ion. This molecule is not neutral; it is an ion with a full negative charge on the carboxylate group. Ionic compounds generally have extremely high solubility in polar solvents like water. The full negative charge on the propanoate ion allows for very strong ion-dipole interactions with the partial positive charges on the hydrogen atoms of surrounding water molecules. These ion-dipole forces are significantly stronger than the hydrogen bonds that the neutral alcohol or carboxylic acids can form. The energy released from forming these strong interactions greatly favors the dissolution of the ion in water. Consequently, the propanoate ion is by far the most soluble compound among the choices
