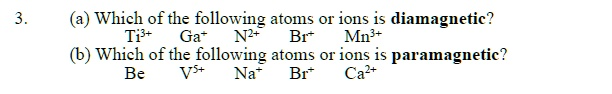
The Correct Answer and Explanation is:
The correct answers are:
(a) Ga⁺
(b) Br⁺
Here is the explanation:
The magnetic properties of an atom or ion depend on its electron configuration. A species is diamagnetic if all its electrons are in paired orbitals. These substances are weakly repelled by an external magnetic field. In contrast, a species is paramagnetic if it has one or more unpaired electrons. The spin of these unpaired electrons creates a small magnetic moment, causing the substance to be attracted to an external magnetic field. To determine the magnetic property, we must find the electron configuration for each species.
(a) Which is diamagnetic?
- Ti³⁺: Titanium (Ti, Z=22) is [Ar] 4s² 3d². The Ti³⁺ ion loses three electrons, resulting in the configuration [Ar] 3d¹. It has one unpaired electron, so it is paramagnetic.
- Ga⁺: Gallium (Ga, Z=31) is [Ar] 4s² 3d¹⁰ 4p¹. The Ga⁺ ion loses its single 4p electron, resulting in the configuration [Ar] 4s² 3d¹⁰. Both the 4s and 3d subshells are completely filled. With no unpaired electrons, Ga⁺ is diamagnetic.
- N²⁺: Nitrogen (N, Z=7) is 1s² 2s² 2p³. The N²⁺ ion is 1s² 2s² 2p¹. It has one unpaired electron in its 2p orbital, so it is paramagnetic.
- Br⁺: Bromine (Br, Z=35) is [Ar] 4s² 3d¹⁰ 4p⁵. The Br⁺ ion is [Ar] 4s² 3d¹⁰ 4p⁴. Its 4p subshell contains two unpaired electrons, making it paramagnetic.
- Mn³⁺: Manganese (Mn, Z=25) is [Ar] 4s² 3d⁵. The Mn³⁺ ion is [Ar] 3d⁴. It has four unpaired electrons, so it is paramagnetic.
(b) Which is paramagnetic?
- Be: Beryllium (Be, Z=4) is 1s² 2s². All electrons are paired, so it is diamagnetic.
- V⁵⁺: Vanadium (V, Z=23) is [Ar] 4s² 3d³. The V⁵⁺ ion loses all five valence electrons, leaving the noble gas configuration [Ar]. All electrons are paired, so it is diamagnetic.
- Na⁺: Sodium (Na, Z=11) loses one electron to form the Na⁺ ion with the configuration [Ne]. All electrons are paired, so it is diamagnetic.
- Br⁺: As determined above, the Br⁺ ion has the configuration [Ar] 4s² 3d¹⁰ 4p⁴, which contains two unpaired electrons. Therefore, Br⁺ is paramagnetic.
- Ca²⁺: Calcium (Ca, Z=20) loses two electrons to form the Ca²⁺ ion with the configuration [Ar]. All electrons are paired, so it is diamagnetic.
