The Correct Answer and Explanation is:
Here are the balanced chemical equations:
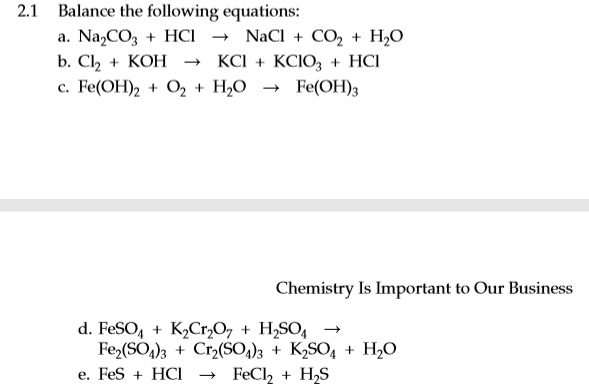
a. Na₂CO₃ + 2HCl → 2NaCl + CO₂ + H₂O
b. 3Cl₂ + 3KOH → 2KCl + KClO₃ + 3HCl
c. 4Fe(OH)₂ + O₂ + 2H₂O → 4Fe(OH)₃
d. 6FeSO₄ + K₂Cr₂O₇ + 7H₂SO₄ → 3Fe₂(SO₄)₃ + Cr₂(SO₄)₃ + K₂SO₄ + 7H₂O
e. FeS + 2HCl → FeCl₂ + H₂S
Explanation of Balancing
Balancing chemical equations is a fundamental process in chemistry that ensures the law of conservation of mass is upheld. This principle dictates that matter cannot be created or destroyed during a chemical reaction. Consequently, the number of atoms for each element must be identical on both the reactant and product sides of an equation. We achieve this balance by adjusting the stoichiometric coefficients, the whole numbers placed before each chemical formula.
For straightforward reactions, balancing by inspection is often sufficient. Consider equation (e), FeS + HCl → FeCl₂ + H₂S. We can see that the iron and sulfur atoms are already balanced. However, the products contain two chlorine atoms and two hydrogen atoms, while the reactants initially show only one of each. By placing a coefficient of 2 in front of the HCl, the equation becomes FeS + 2HCl → FeCl₂ + H₂S, satisfying the atom count for all elements. The same inspection method can be applied to equation (a).
More complex reactions, such as (c), Fe(OH)₂ + O₂ + H₂O → Fe(OH)₃, benefit from a systematic algebraic approach. By assigning variable coefficients to each substance, we can set up a system of linear equations based on the atom count for each element. Solving these equations provides the simplest whole number ratio for the coefficients, which is 4:1:2:4 in this case.
For complex oxidation reduction (redox) reactions like (d), the half reaction method is the most reliable. This involves separating the overall reaction into an oxidation half reaction (loss of electrons) and a reduction half reaction (gain of electrons). Each half reaction is independently balanced for atoms and charge. The key step is to equalize the number of electrons transferred by multiplying the half reactions by appropriate integers. Finally, the balanced half reactions are combined, and any spectator ions are reintroduced to yield the final, fully balanced molecular equation.
