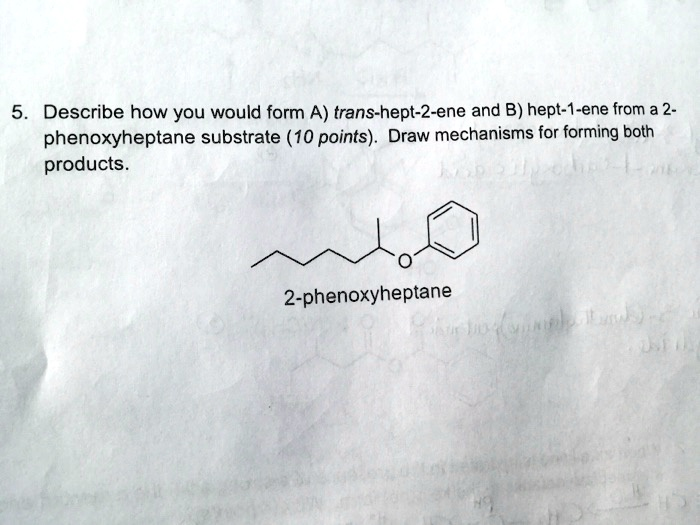
Describe how you would form A) trans-hept-2-ene and B) hept-1-ene from a 2- phenoxyheptane substrate (10 points). Draw mechanisms for forming both products. 2-phenoxyheptane
The Correct Answer and Explanation is:
Of course. Here is a description of the reactions and the mechanisms for forming both products from 2-phenoxyheptane.
This problem requires performing an elimination reaction on the 2-phenoxyheptane substrate. The phenoxy group (-OPh) on carbon 2 will act as the leaving group, and a proton will be removed from an adjacent carbon (either C1 or C3). The choice of base is critical for controlling which product is formed. This is a classic example of controlling the regioselectivity of an E2 elimination reaction to favor either the Zaitsev (more substituted) or Hofmann (less substituted) product.
A) Formation of trans-hept-2-ene (Zaitsev Product)
To form the more substituted and thermodynamically more stable alkene, trans-hept-2-ene, we need to promote Zaitsev’s rule. This is achieved by using a strong, sterically unhindered (small) base. A suitable choice would be sodium ethoxide (NaOEt) in ethanol (EtOH) as the solvent, often with the application of heat to facilitate the reaction.
The small ethoxide ion can easily access the proton on the more sterically hindered internal carbon (C3). The E2 mechanism requires an anti-periplanar alignment of the proton on C3 and the phenoxy leaving group on C2. This specific geometric arrangement leads preferentially to the formation of the trans isomer, which is more stable than the cis isomer.
Mechanism for trans-hept-2-ene:
- The ethoxide ion (EtO⁻) acts as a base and removes a proton from carbon 3.
- In a single, concerted step, the electrons from the C-H bond move to form a new pi bond between carbons 2 and 3.
- Simultaneously, the carbon-oxygen bond breaks, and the phenoxide ion (PhO⁻) departs as the leaving group.
B) Formation of hept-1-ene (Hofmann Product)
To form the less substituted alkene, hept-1-ene, we need to promote Hofmann’s rule. This is achieved by using a strong, sterically hindered (bulky) base. The classic choice for this is potassium tert-butoxide (KOt-Bu) in tert-butanol (t-BuOH) as the solvent.
The large tert-butoxide ion has difficulty reaching the more substituted C3 proton due to steric hindrance from the alkyl chain. It is much easier for the bulky base to access the more exposed protons on the terminal methyl group (C1). Therefore, proton removal from C1 is kinetically favored, leading to the formation of the less substituted alkene, hept-1-ene.
Mechanism for hept-1-ene:
- The bulky tert-butoxide ion (t-BuO⁻) acts as a base and removes a proton from the terminal methyl group (carbon 1).
- In a concerted E2 mechanism, the electrons from the C-H bond swing down to form the pi bond between carbons 1 and 2.
- Simultaneously, the phenoxide leaving group departs from carbon 2.
