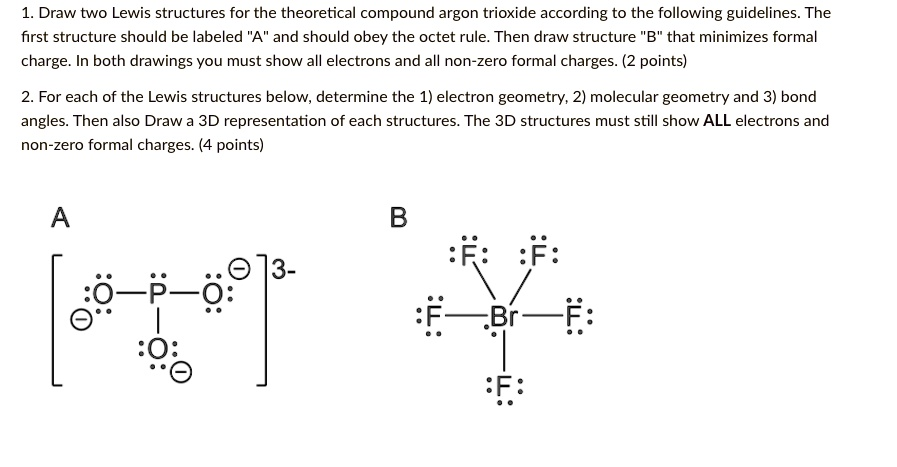
Draw two Lewis structures for the theoretical compound argon trioxide according to the following guidelines The first structure should be labeled “A” and should obey the octet rule_ Then draw structure B” that minimizes formal charge In both drawings you must show all electrons and all non-zero formal charges. (2 points) For each of the Lewis structures below, determine the 1) electron geometry; 2) molecular geometry and 3) bond angles: Then also Draw 3D representation of each structures. The 3D structures must still show ALL electrons and non-zero formal charges. (4 points) B R: :F: 73- ~F_&: E~Br
The Correct Answer and Explanation is:
Let’s break down the task and explain how to approach the Lewis structures for argon trioxide (ArO₃), as well as the subsequent steps.
- Drawing the Lewis Structures:
- Structure A (Octet rule):
Since argon (Ar) is a noble gas, it generally doesn’t form bonds. However, in this compound, it’s assumed that argon can form bonds with oxygen in a structure. Each oxygen atom in this structure should follow the octet rule. To obey the octet rule, the oxygen atoms would likely each form a single bond with argon (Ar). The formal charges of each atom should be calculated as we draw the structure. - Structure B (Minimizing Formal Charges):
In structure B, you should adjust the number of bonds to minimize formal charges. This may involve double bonds between argon and some oxygen atoms. The goal is to ensure that the formal charges are as close to zero as possible, which generally leads to a more stable structure.
- Structure A (Octet rule):
- Electron Geometry, Molecular Geometry, and Bond Angles: After determining the Lewis structures, we will focus on the molecular shape and bond angles:
- Electron Geometry refers to the arrangement of bonding and non-bonding electron pairs around the central atom.
- Molecular Geometry describes the shape of the molecule considering only the atoms.
- Bond Angles describe the angles between adjacent bonds in the structure.
- Electron Geometry: Likely linear or trigonal planar, depending on the number of bonds and lone pairs around argon.
- Molecular Geometry: Similar to the electron geometry, but adjusted based on the presence of lone pairs or bonding.
- Bond Angles: Approximately 120° if it’s trigonal planar.
- Electron Geometry: Likely tetrahedral or trigonal bipyramidal if there are multiple bonds.
- Molecular Geometry: Adjusted similarly, but with emphasis on formal charge minimization.
- Bond Angles: Generally close to 109.5° (for tetrahedral) or 90°/120° (for trigonal bipyramidal).
- 3D Representation:
These 3D structures must show the correct bonding angles, formal charges, and lone pairs. For Structure A and B, 3D representations should reflect bond angles and lone pairs around the central atom.
