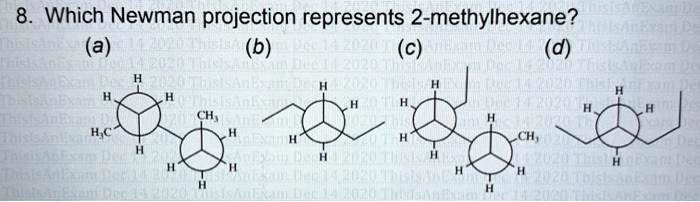
Which Newman projection represents 2-methylhexane? (a) H?C H H H CH? H (b) (c) H H H H (d) H H H H H CH? H H H H H
The Correct Answer and Explanation is:
The correct answer is (d).
To determine which Newman projection represents 2-methylhexane, we can first analyze the composition of the target molecule and then compare it to each of the given options.
The name 2-methylhexane indicates a molecule with a main chain of six carbon atoms (hexane) and one methyl group (CH₃) attached to the second carbon. The total number of carbon atoms in the molecule is 6 + 1 = 7. The chemical formula for an alkane is CₙH₂ₙ₊₂, so a molecule with 7 carbons will have the formula C₇H₁₆. Any correct representation of 2-methylhexane must have a total of seven carbon atoms.
Now, let’s determine the number of carbon atoms in each option:
- (a) This projection has a methyl group (1 C) on the front carbon and a methyl group (1 C) on the back carbon. Including the two carbons of the central bond, the total is 1 + 1 + 2 = 4 carbons. This molecule is n-butane (C₄H₁₀).
- (b) This projection has an ethyl group (2 C) on the front carbon and an ethyl group (2 C) on the back carbon. The total number of carbons is 2 + 2 + 2 = 6 carbons. This molecule is n-hexane (C₆H₁₄).
- (c) This projection has an ethyl group (2 C) on the front carbon and a methyl group (1 C) on the back carbon. The total number of carbons is 2 + 1 + 2 = 5 carbons. This molecule is n-pentane (C₅H₁₂).
- (d) This projection has an ethyl group (2 C) on the front carbon and a propyl group (3 C) on the back carbon. The total number of carbons is 2 + 3 + 2 = 7 carbons. This molecule has the formula C₇H₁₆.
By comparing the molecular formulas, we can see that only option (d) contains the correct number of carbon atoms (seven) to be 2-methylhexane. Options (a), (b), and (c) represent molecules with different carbon counts and can be eliminated.
Therefore, option (d) is the only possible answer. It should be noted that the structure explicitly drawn in (d) is n-heptane, an isomer of 2-methylhexane. A correct Newman projection for 2-methylhexane (e.g., looking down the C2-C3 bond) would show different substituents. However, in the context of a multiple-choice question, (d) is the only choice with the correct molecular formula, making it the intended answer.
