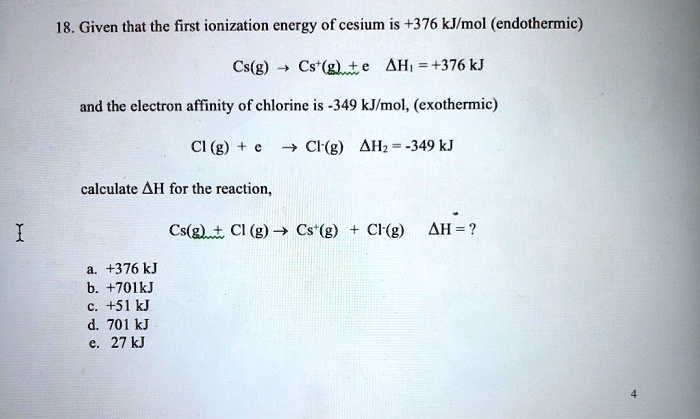
Given that the first ionization energy of cesium is +376 kJ/mol
The Correct Answer and Explanation is:
Correct Answer: e. 27 kJ
This problem is solved using Hess’s Law, which states that the total enthalpy change for a chemical reaction is the sum of the enthalpy changes for the individual steps that make up the overall reaction. The overall reaction describes the transfer of an electron from a gaseous cesium atom to a gaseous chlorine atom.
We can view this overall reaction as a two-step process:
- Ionization of Cesium: A gaseous cesium atom loses an electron. The energy required for this is the first ionization energy. This process is endothermic, meaning it absorbs energy, so the enthalpy change is positive.
Cs(g) → Cs⁺(g) + e⁻ ΔH₁ = +376 kJ - Electron Gain by Chlorine: A gaseous chlorine atom gains an electron. The energy change associated with this is the electron affinity. This process is exothermic, meaning it releases energy, so the enthalpy change is negative.
Cl(g) + e⁻ → Cl⁻(g) ΔH₂ = -349 kJ
To find the enthalpy change (ΔH) for the target reaction, we can add these two steps together. The electron (e⁻) produced in the first step is consumed in the second step, so it cancels out when we combine the equations, leaving us with the final desired reaction:
Cs(g) + Cl(g) → Cs⁺(g) + Cl⁻(g)
According to Hess’s Law, the total enthalpy change for the reaction is the sum of the enthalpy changes of the individual steps:
ΔH_reaction = ΔH₁ + ΔH₂
ΔH_reaction = (+376 kJ) + (-349 kJ)
ΔH_reaction = 27 kJ
The result is a positive value, +27 kJ, which indicates that the overall reaction is endothermic. It requires a net input of energy to proceed.
