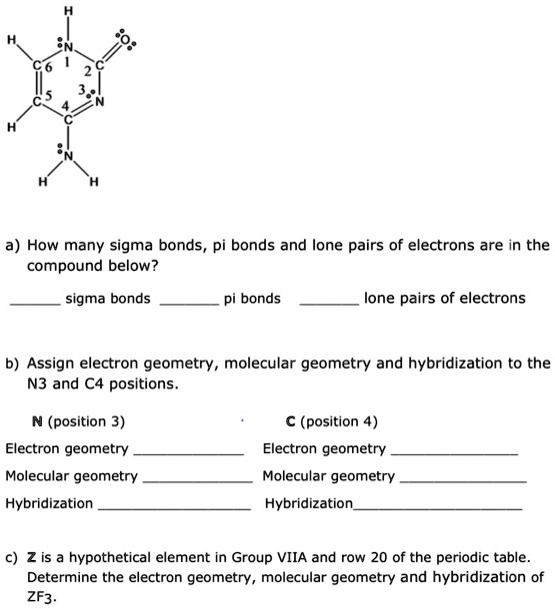
How many sigma bonds, pi bonds, and lone pairs of electrons are in the compound below? Sigma bonds Pi bonds Lone pairs of electrons Assign electron geometry, molecular geometry, and hybridization to the N3 and C4 positions. N (position 3) C (position 4) Electron geometry Molecular geometry Hybridization Electron geometry Molecular geometry Hybridization c) Element 2 is a hypothetical element in Group VIIA and row 20 of the periodic table. Determine the electron geometry, molecular geometry, and hybridization of ZF3.
The Correct Answer and Explanation is
To answer your questions systematically, let’s break them down:
Part 1: Sigma bonds, Pi bonds, and Lone pairs
For the compound you’re referring to (which seems to have Nitrogen in position 3 and Carbon in position 4), let’s clarify what types of bonds and lone pairs are involved:
- Sigma bonds: These are single bonds where electrons are shared in a region directly between the two atoms. Each bond between atoms will involve one sigma bond.
- Pi bonds: These occur when two atomic orbitals overlap side-by-side. These are often seen in double or triple bonds.
- Lone pairs: These are pairs of electrons that are localized on a single atom and are not involved in bonding.
If you can provide the structure of the compound or clarify which atoms are bonded to each other, I can give you a precise count of the sigma bonds, pi bonds, and lone pairs.
Part 2: Electron Geometry, Molecular Geometry, and Hybridization for N3 and C4 positions
- N (Position 3):
- Electron Geometry: The electron geometry depends on the number of regions of electron density (bonds and lone pairs) around the nitrogen. Typically, if nitrogen is part of a trigonal planar or tetrahedral structure, its electron geometry will align accordingly.
- Molecular Geometry: This refers to the shape formed by the atoms (excluding lone pairs).
- Hybridization: For nitrogen, if it forms three bonds (as in NH3), it’s sp³ hybridized; for two bonds (like N₂), it could be sp hybridized.
- C (Position 4):
- Electron Geometry: If carbon has four bonds, it will have a tetrahedral electron geometry (sp³ hybridized). If it has two bonds, it will be linear (sp hybridized).
- Molecular Geometry: This will be tetrahedral for sp³ hybridization or linear for sp hybridization.
- Hybridization: This will depend on the number of bonds; for example, if C is bonded to four groups, it will be sp³ hybridized.
Part 3: Electron Geometry, Molecular Geometry, and Hybridization of ZF3
- Element Z (Group VIIA, Row 20) and Fluorine (F):
- Electron Geometry: Assuming Z forms three bonds with F, the electron geometry will likely be trigonal planar (if Z has no lone pairs), or trigonal pyramidal if it has one lone pair.
- Molecular Geometry: If Z has no lone pairs, the geometry would be trigonal planar. If there’s one lone pair, it would be trigonal pyramidal.
- Hybridization: For three bonds and no lone pairs, Z would be sp² hybridized. If there is one lone pair, Z would also be sp² hybridized since the electron pairs dictate the geometry.
