Identify the monomer used to make the following polymer. CO?H CO?H CH?=CHCO?H CO?H CH?CO?H CH?CH?CO?H CH?CH?CH?CO?H I II III IV
The Correct Answer and Explanation is:
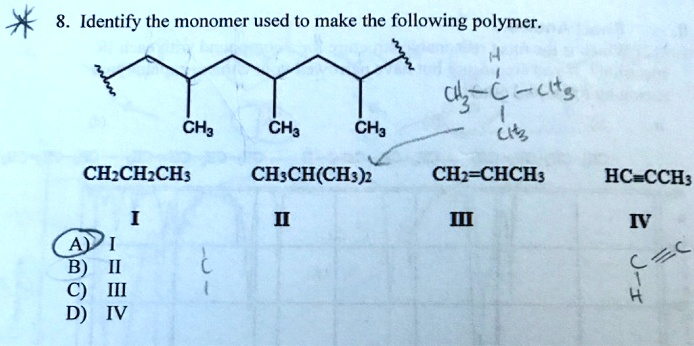
The correct answer is C, which corresponds to molecule III.
The image displays an addition polymer and asks to identify the monomer from which it was made. Here is a step-by-step explanation of how to determine the correct monomer.
First, we need to analyze the structure of the polymer. The polymer shown is poly(propene), also known as polypropylene. It is characterized by a long saturated carbon backbone with methyl (CH₃) groups attached to every other carbon atom. The key to finding the monomer is to identify the repeating structural unit within the polymer chain. Looking at the structure, we can see that the pattern -[CH(CH₃)-CH₂]- repeats itself along the chain. This two-carbon unit is the building block of the polymer.
Second, we must understand the process of addition polymerization. This type of polymerization involves monomers, typically alkenes, which have a carbon-carbon double bond. During the reaction, the weaker pi (π) bond of the double bond breaks, and the electrons are used to form new, strong sigma (σ) bonds with adjacent monomer units, linking them together into a long chain.
To find the original monomer, we need to mentally reverse this process. We take the repeating unit we identified, -[CH(CH₃)-CH₂]-, and re-form the double bond between the two carbon atoms that constitute the polymer’s backbone. This action converts the two single bonds linking the units back into a double bond within a single monomer molecule.
When we re-form the double bond in the -[CH(CH₃)-CH₂]- unit, we get the molecule CH(CH₃)=CH₂. This is more commonly written as CH₂=CHCH₃, which is the chemical structure for propene.
Finally, we compare this derived monomer with the given options:
- I: CH₂CH₂CH₃ is propane, an alkane. It has no double bond and cannot undergo addition polymerization.
- II: CH₃CH(CH₃)₂ is 2-methylpropane, also an alkane, and cannot be the monomer.
- III: CH₂=CHCH₃ is propene, an alkene. This structure perfectly matches the monomer we determined is necessary to form the given polymer.
- IV: HC≡CCH₃ is propyne, an alkyne. Its polymerization would result in a polymer with a different structure, containing double bonds in the backbone.
Therefore, the correct monomer used to make the polymer is propene (III), making option C the correct answer.
