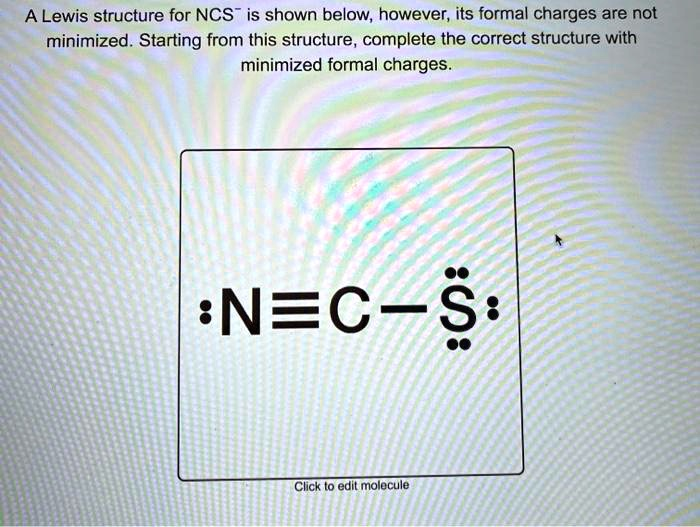
A Lewis structure for NCS is shown below, however, its formal charges are not minimized
The Correct Answer and Explanation is:
The correct Lewis structure for the thiocyanate ion (NCS⁻) with minimized formal charges is:
[:N̈=C=S̈:]⁻
This structure shows a double bond between the nitrogen and carbon atoms and a double bond between the carbon and sulfur atoms. The nitrogen atom has two lone pairs of electrons, and the sulfur atom also has two lone pairs.
Explanation
To determine the most stable Lewis structure for an ion like thiocyanate (NCS⁻), we need to find the arrangement of electrons that results in the most minimized formal charges. The best structure is the one where formal charges are closest to zero, and any negative formal charge resides on the most electronegative atom.
First, we calculate the total number of valence electrons for NCS⁻:
- Nitrogen (N) is in Group 15 and has 5 valence electrons.
- Carbon (C) is in Group 14 and has 4 valence electrons.
- Sulfur (S) is in Group 16 and has 6 valence electrons.
- The negative charge of the ion adds 1 electron.
Total valence electrons = 5 + 4 + 6 + 1 = 16 electrons.
The initial structure provided is [:N≡C-S̈:]⁻, with a triple bond between N and C, a single bond between C and S, one lone pair on N, and three lone pairs on S. Let’s calculate the formal charges for this structure:
- Formal Charge of N: 5 – 2 (lone pair) – 3 (bonds) = 0
- Formal Charge of C: 4 – 0 (lone pair) – 4 (bonds) = 0
- Formal Charge of S: 6 – 6 (lone pairs) – 1 (bond) = -1
This structure has a formal charge of -1 on the sulfur atom.
Now, let’s consider the correct structure, [:N̈=C=S̈:]⁻, which has double bonds between all atoms and two lone pairs on both N and S. Calculating its formal charges:
- Formal Charge of N: 5 – 4 (lone pairs) – 2 (bonds) = -1
- Formal Charge of C: 4 – 0 (lone pair) – 4 (bonds) = 0
- Formal Charge of S: 6 – 4 (lone pairs) – 2 (bonds) = 0
When comparing these two structures, both satisfy the octet rule for all atoms and have a net charge of -1. However, the stability is determined by the location of the negative formal charge. The electronegativity values are N (3.04), S (2.58), and C (2.55). Since nitrogen is the most electronegative atom in the ion, the structure that places the negative formal charge on nitrogen is the most stable and significant resonance contributor. Therefore, the structure with two double bonds is the preferred Lewis structure because it correctly places the negative charge on the more electronegative nitrogen atom, better reflecting the true electron distribution in the thiocyanate ion.
