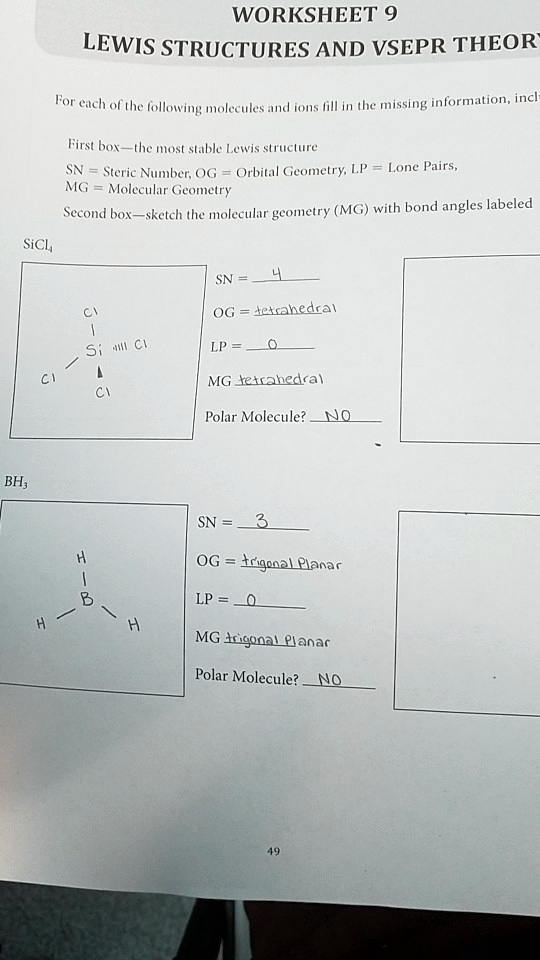
WORKSHEET 9 LEWIS STRUCTURES AND VSEPR THEORY For each of the following molecules and ions fill in the missing information, including First box—the most stable Lewis structure SN = Steric Number, OG = Orbital Geometry, LP = Lone Pairs, MG = Molecular Geometry Second box—sketch the molecular geometry (MG) with bond angles labeled SiCl
SN = 4 Cl OG = tetrahedral Si Cl LP = 0 Cl Cl MG tetrahedral Polar Molecule? NO BH
H B H H SN = 3 OG = trigonal Planar LP = 0 MG trigonal Planar Polar Molecule? NO 49
The Correct Answer and Explanation is:
Based on the provided worksheet, the handwritten answers are all correct. The only missing information is the sketch of the molecular geometry with bond angles for each molecule.
Here is the information needed to complete the worksheet:
For SiCl₄:
The second box should contain a sketch of a tetrahedral molecule. Silicon (Si) is the central atom. The four chlorine (Cl) atoms are positioned at the vertices of a tetrahedron around the silicon. The bond angle between any two Cl-Si-Cl bonds should be labeled as 109.5°.
For BH₃:
The second box should contain a sketch of a trigonal planar molecule. Boron (B) is the central atom. The three hydrogen (H) atoms are positioned at the corners of an equilateral triangle around the boron, all in the same plane. The bond angle between any two H-B-H bonds should be labeled as 120°.
Explanation
The provided worksheet correctly applies VSEPR (Valence Shell Electron Pair Repulsion) theory to determine the geometry of SiCl₄ and BH₃.
For silicon tetrachloride (SiCl₄), the central silicon atom has 4 valence electrons and each of the four chlorine atoms has 7, for a total of 32 valence electrons. The Lewis structure places silicon at the center, forming single bonds with each of the four chlorine atoms. This uses 8 electrons for bonding, and the remaining 24 electrons are placed as three lone pairs on each chlorine atom, satisfying every atom’s octet. The central silicon atom has four bonding pairs and zero lone pairs. This gives a steric number of 4, corresponding to a tetrahedral electron geometry. Since there are no lone pairs on the central atom, the molecular geometry is also tetrahedral. The symmetrical arrangement of the polar Si-Cl bonds causes their individual dipoles to cancel out, making the SiCl₄ molecule nonpolar. The ideal bond angle in a perfect tetrahedral shape is 109.5°.
For borane (BH₃), the central boron atom has 3 valence electrons and each of the three hydrogen atoms has 1, for a total of 6 valence electrons. Boron is the central atom, forming single bonds with the three hydrogen atoms. This structure uses all 6 valence electrons. Boron is a common exception to the octet rule, being stable with only six electrons in its valence shell. The central boron atom has three bonding pairs and zero lone pairs, resulting in a steric number of 3. This corresponds to a trigonal planar electron and molecular geometry. The molecule is flat with the hydrogen atoms spread out as far as possible, creating 120° bond angles between them. This symmetrical geometry ensures that any minor polarity in the B-H bonds would cancel, rendering the BH₃ molecule nonpolar.
