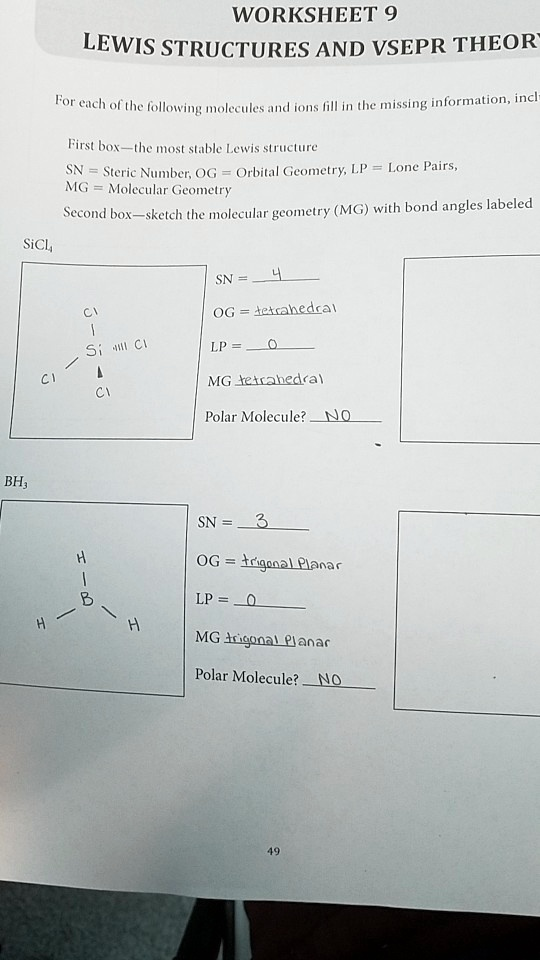
WORKSHEET 9 LEWIS STRUCTURES AND VSEPR THEORY For each of the following molecules and ions fill in the missing information, including First box—the most stable Lewis structure SN = Steric Number, OG = Orbital Geometry, LP = Lone Pairs, MG = Molecular Geometry Second box—sketch the molecular geometry (MG) with bond angles labeled SiCl
SN = 4 Cl OG = tetrahedral Si Cl LP = 0 Cl Cl MG tetrahedral Polar Molecule? NO BH
H B H H SN = 3 OG = trigonal Planar LP = 0 MG trigonal Planar Polar Molecule? NO 49
The Correct Answer and Explanation is:
Based on the provided worksheet, the answers for the BH3 (borane) molecule are correct, however, the sketch of the molecular geometry with labeled bond angles is missing. Here is the complete and correct information for BH3.
Molecule: BH₃
- Most Stable Lewis Structure: A central Boron (B) atom single bonded to three Hydrogen (H) atoms. Boron does not have any lone pairs.
- SN (Steric Number): 3
- OG (Orbital Geometry): Trigonal Planar
- LP (Lone Pairs): 0
- MG (Molecular Geometry): Trigonal Planar
- Polar Molecule?: NO
- Sketch of Molecular Geometry: A central Boron atom with three Hydrogen atoms arranged symmetrically around it in a flat, triangular shape. The H-B-H bond angle is 120°.
Explanation
The analysis of the borane molecule, BH₃, begins with determining its total valence electrons. Boron, from Group 13, contributes 3 valence electrons, and each of the three hydrogen atoms contributes 1, for a total of 6 valence electrons. The Lewis structure is drawn with boron as the central atom, single bonded to each hydrogen atom. This uses all 6 valence electrons (3 bonds x 2 electrons). Boron is a known exception to the octet rule and is stable with only 6 electrons in its valence shell, so this structure is correct and there are no lone pairs on the central boron atom.
Next, we apply VSEPR theory. The steric number (SN) is the sum of bonding domains and lone pairs around the central atom. For BH₃, there are 3 bonding domains (the B-H bonds) and 0 lone pairs, making the SN equal to 3.
A steric number of 3 corresponds to a trigonal planar orbital geometry, which describes the arrangement of all electron domains. Since there are no lone pairs (LP = 0), the molecular geometry, which describes the arrangement of only the atoms, is identical to the orbital geometry. Therefore, the molecular geometry of BH₃ is also trigonal planar.
In a trigonal planar arrangement, the electron domains position themselves as far apart as possible to minimize repulsion, resulting in ideal bond angles of 120°.
Finally, to determine molecular polarity, we consider both bond polarity and molecular shape. The electronegativity difference between boron (2.04) and hydrogen (2.20) is very small, making the B-H bond nearly nonpolar. Even if we consider the small bond dipoles, the molecule’s perfectly symmetrical trigonal planar shape causes these dipoles to cancel each other out. The result is a net dipole moment of zero, making BH₃ a nonpolar molecule.
