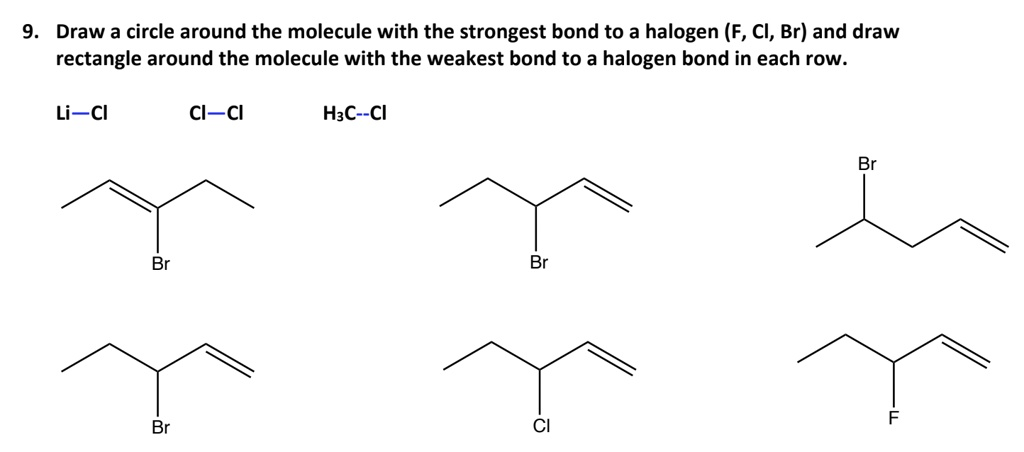
. Draw a circle around the molecule with the strongest bond to a halogen (F, Cl, Br) and draw rectangle around the molecule with the weakest bond to a halogen bond in each row. Li\text{-}Cl Cl\text{-}Cl H_3C\text{-}Cl
The Correct Answer and Explanation is:
To analyze the bonds to halogens in the compounds Li-Cl, Cl-Cl, and H₃C-Cl, we need to understand the factors that influence bond strength. These factors include bond length, atomic size, and electronegativity.
- Li-Cl (Lithium chloride):
- In Li-Cl, lithium has a lower electronegativity compared to chlorine. The lithium ion (Li⁺) is relatively large, which leads to a longer bond length. This results in a weaker bond compared to bonds involving smaller atoms like hydrogen or carbon.
- Cl-Cl (Chlorine molecule):
- In Cl-Cl, both atoms are chlorine. The Cl-Cl bond is a nonpolar covalent bond with moderate bond strength. The bond length is relatively short, and there is no difference in electronegativity between the two chlorine atoms, which makes this bond weaker than a typical bond involving a halogen and a metal.
- H₃C-Cl (Chloromethane):
- The bond in H₃C-Cl is a polar covalent bond because carbon is more electronegative than hydrogen, but less so than chlorine. As a result, the bond is stronger than Li-Cl but weaker than Cl-Cl because the electronegativity difference is moderate. The bond length is shorter than that in Li-Cl but still longer than a bond between two chlorine atoms.
Conclusion:
- The strongest bond to a halogen is in H₃C-Cl, because carbon and chlorine form a moderately strong polar covalent bond, and the size of the atoms involved is intermediate.
- The weakest bond to a halogen is in Li-Cl, because lithium, a metal, has a relatively large ionic radius and low electronegativity, which results in a weaker ionic bond with chlorine.
Thus, in each row:
- Circle H₃C-Cl for the strongest bond to a halogen.
- Draw a rectangle around Li-Cl for the weakest bond to a halogen.
