Name the alkene: CH3CH2CH=CH2
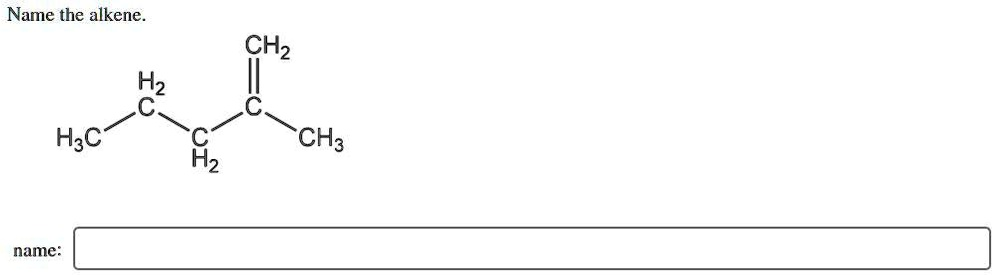
The Correct Answer and Explanation is:
2-methylpent-1-ene
To determine the correct IUPAC name for the alkene shown, we must follow a systematic set of rules.
- Identify the Parent Chain: The first and most crucial step is to find the longest continuous carbon chain that incorporates both carbons of the carbon-carbon double bond (C=C). The structure in the image is CH₃-CH₂-CH₂-C(=CH₂)-CH₃. The double bond involves the central carbon atom and the CH₂ group attached to it.
- One possible chain containing the double bond is CH₂=C-CH₃. This chain is only three carbons long.
- A longer chain can be traced from the double bond into the propyl group: CH₂=C-CH₂-CH₂-CH₃. This chain is five carbons long.
According to IUPAC rules, we must choose the longest chain, which is the five-carbon chain. Therefore, the base name of the molecule is derived from “pentane,” making it a “pentene.”
- Number the Parent Chain: The parent chain must be numbered to give the carbons of the double bond the lowest possible locants (positions). The five-carbon chain is CH₂=C-CH₂-CH₂-CH₃. To give the double bond the lowest number, we must start numbering from the end closest to it. In this case, we start from the =CH₂ group.
- The =CH₂ carbon is C1.
- The next carbon in the double bond is C2.
- The rest of the chain follows: -CH₂(C3)-CH₂(C4)-CH₃(C5).
Since the double bond starts at C1, the parent alkene is named pent-1-ene.
- Identify and Locate Substituents: After identifying and numbering the parent chain, we look for any alkyl groups (substituents) attached to it. In the given structure, there is a methyl group (-CH₃) attached to the C2 position of our numbered parent chain. Therefore, the substituent is identified as 2-methyl.
- Assemble the Full Name: The final IUPAC name is constructed by combining the substituent name and location with the parent alkene name. The standard format is: (substituent location)-(substituent name)(parent name).
Putting the pieces together, we get 2-methylpent-1-ene.
