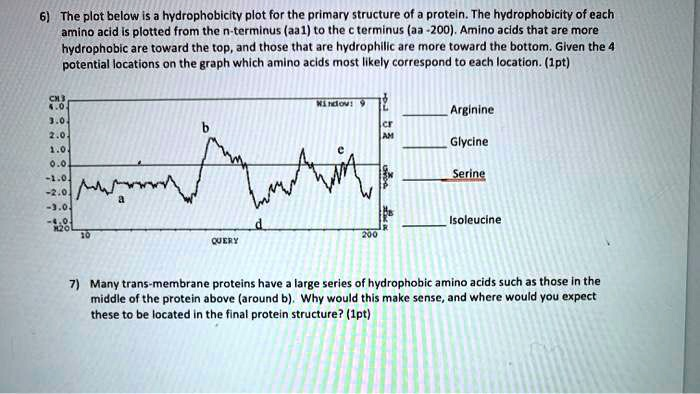
The plot below is a hydrophobicity plot for the primary structure of a protein. The hydrophobicity of each amino acid is plotted from the N-terminus (aa1) to the C-terminus (aa2001). Amino acids that are more hydrophobic are towards the top, and those that are hydrophilic are towards the bottom. Given the potential locations on the graph, which amino acids most likely correspond to each location? (1pt) Arginine Glycine Serine Alanine Isoleucine Tyrosine Many transmembrane proteins have large series of hydrophobic amino acids, such as those in the middle of the protein above (around b). Why would this make sense, and where would you expect these to be located in the final protein structure? (1pt)
The Correct Answer and Explanation is:
To analyze this hydrophobicity plot and determine the amino acids corresponding to the locations, we need to evaluate the hydrophobicity of the given amino acids based on their chemical properties.
- Arginine (Arg): Arginine is a polar amino acid due to its positively charged guanidino group. It would be expected to be located towards the bottom of the plot, where hydrophilic amino acids are found.
- Glycine (Gly): Glycine is a small, non-polar amino acid. While it lacks a large hydrophobic side chain, it can be considered relatively neutral or hydrophilic. It would likely be towards the lower part of the plot but slightly higher than strongly hydrophilic amino acids.
- Serine (Ser): Serine is a polar amino acid with a hydroxyl group. This makes it hydrophilic, so it should be placed towards the lower part of the plot.
- Alanine (Ala): Alanine is a small, hydrophobic amino acid with a methyl group (-CH₃) side chain. It is typically located higher on the plot, indicating moderate hydrophobicity.
- Isoleucine (Ile): Isoleucine is a branched-chain, non-polar, hydrophobic amino acid. It would be found towards the top of the plot, indicating its hydrophobic nature.
- Tyrosine (Tyr): Tyrosine has both hydrophobic and hydrophilic characteristics due to its aromatic ring and hydroxyl group. It would likely be located in a moderate position, higher than serine but not as high as isoleucine or alanine.
Why do many transmembrane proteins have large series of hydrophobic amino acids?
Transmembrane proteins span the lipid bilayer of cell membranes. The interior of the membrane is hydrophobic, which favors the insertion of hydrophobic amino acids into this region. Hydrophobic amino acids, such as isoleucine and alanine, interact favorably with the lipid bilayer, enabling the protein to integrate into the membrane.
Final location in the protein structure:
The hydrophobic amino acids are most likely to be located in the transmembrane region of the protein. These regions are embedded in the membrane, where the hydrophobic side chains interact with the fatty acid tails of the lipid molecules. On the other hand, hydrophilic amino acids, like arginine and serine, are typically located on the exterior of the protein, facing the aqueous environment inside or outside the cell.
The large cluster of hydrophobic residues in the middle of the protein would suggest a transmembrane domain, which helps anchor the protein within the membrane.
