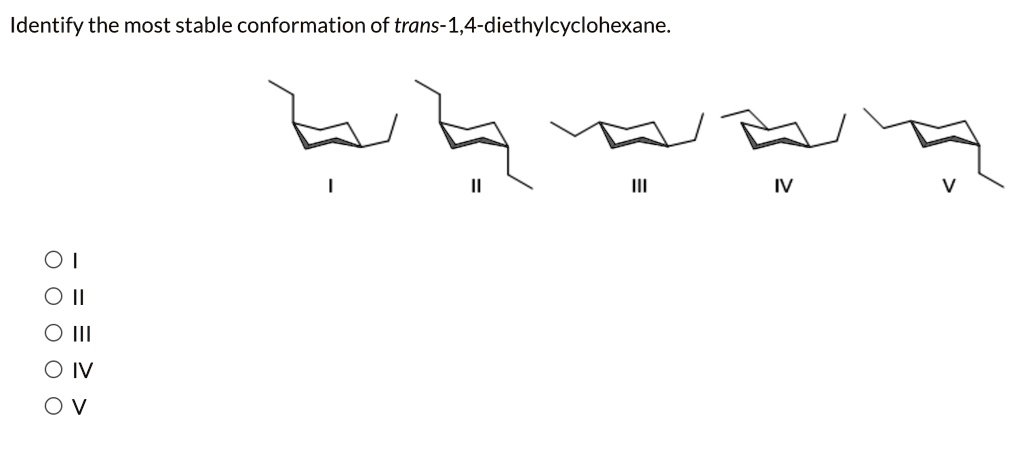
Identify the most stable conformation of trans-1,4-diethylcyclohexane. ?? O II O III O IV O V
The Correct Answer and Explanation is:
The correct answer is II.
Here is a detailed explanation:
The stability of a substituted cyclohexane molecule is primarily determined by its conformation, with the chair conformation being the most stable form. Within the chair conformation, the positions of the substituents play a crucial role. There are two types of positions for substituents: axial (pointing vertically up or down, parallel to the axis of the ring) and equatorial (pointing outwards from the perimeter of the ring).
Substituents, particularly bulky groups like the ethyl group (-CH2CH3), are significantly more stable in the equatorial position. When a bulky group is in the axial position, it experiences steric hindrance from the other two axial atoms (usually hydrogens) on the same side of the ring. This unfavorable interaction, known as a 1,3-diaxial interaction, destabilizes the molecule. Placing the substituent in the more spacious equatorial position minimizes this steric strain.
The term trans-1,4-diethylcyclohexane specifies the relative orientation of the two ethyl groups. For a 1,4-disubstituted cyclohexane, the trans isomer can exist in two interconverting chair conformations:
- A conformation where both ethyl groups are in axial positions (diaxial).
- A conformation where both ethyl groups are in equatorial positions (diequatorial).
To find the most stable conformation, we must compare these two possibilities. The diequatorial conformation is vastly more stable than the diaxial one. In the diequatorial conformer, both bulky ethyl groups occupy the sterically favored equatorial positions, avoiding any significant 1,3-diaxial interactions. Conversely, in the diaxial conformer, both ethyl groups suffer from severe steric strain, making it a very high-energy and unstable state.
Now let’s analyze the given options:
- Structure I: Shows a chair conformation with both ethyl groups in axial positions (one pointing up, one pointing down). This is the unstable diaxial conformer of trans-1,4-diethylcyclohexane.
- Structure II: Shows a chair conformation with both ethyl groups in equatorial positions. This is the highly stable diequatorial conformer of trans-1,4-diethylcyclohexane.
- Structures III and V: Show conformations where one ethyl group is axial and the other is equatorial. This arrangement corresponds to the cis isomer, not the trans isomer.
- Structure IV: Also depicts a cis isomer (one axial, one equatorial).
Therefore, the most stable conformation for trans-1,4-diethylcyclohexane is the one where both ethyl groups are in the equatorial position, which is correctly depicted in Structure II.
