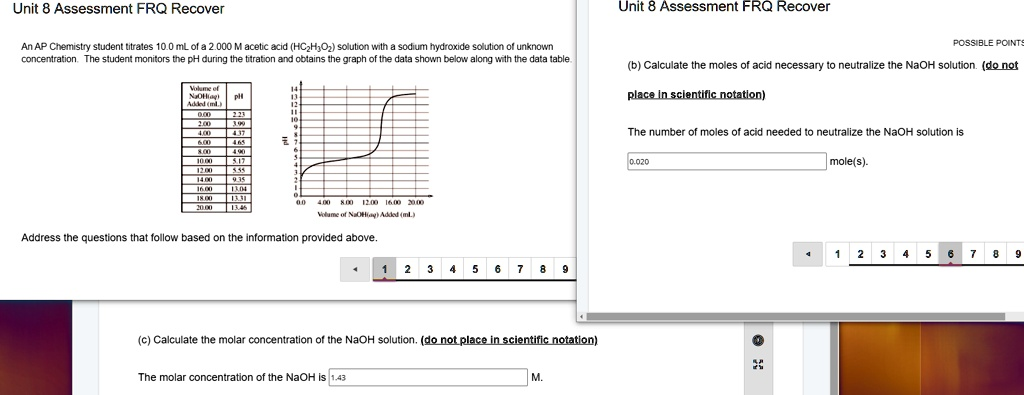
Unit 8 Assessment FRQ Recovery An AP Chemistry student titrates 10.0 mL of a hydrochloric acid (HCl) solution with an unknown concentration with a sodium hydroxide (NaOH) solution. The student monitors the pH of the solution and records the data in the table below. Possible Points (b) Calculate the moles of acid necessary to neutralize the NaOH solution (do not place in scientific notation). The number of moles of acid needed to neutralize the NaOH solution is 0.00 mole(s). Answer the following questions based on the information provided above. Calculate the molar concentration of the NaOH solution (do not place in scientific notation). The molar concentration of the NaOH solution is [missing data].
The Correct Answer and Explanation is:
Here are the correct answers and the detailed explanation for the chemistry problem.
Correct Answers:
(b) Calculate the moles of acid necessary to neutralize the NaOH solution. (do not place in scientific notation)
The number of moles of acid needed to neutralize the NaOH solution is 0.0200 mole(s).
(c) Calculate the molar concentration of the NaOH solution. (do not place in scientific notation)
The molar concentration of the NaOH is 1.43 M.
Explanation
This problem involves a chemical process called a titration, where a solution of known concentration is used to determine the concentration of another solution. Here, a sodium hydroxide (NaOH) solution is used to titrate an acetic acid (HC₂H₃O₂) solution.
Part (b): Calculating the Moles of Acid
The first step is to calculate the initial number of moles of acetic acid present in the flask before the titration begins. The problem states that we start with 10.0 mL of a 2.000 M acetic acid solution. The formula to find moles from molarity and volume is:
Moles = Molarity (M) × Volume (L)
First, we must convert the volume of the acid from milliliters (mL) to liters (L) because molarity is expressed in moles per liter.
Volume = 10.0 mL × (1 L / 1000 mL) = 0.0100 L
Now, we can calculate the moles of acetic acid:
Moles of HC₂H₃O₂ = 2.000 mol/L × 0.0100 L = 0.0200 mol
This value represents the amount of acid that will be completely neutralized by the sodium hydroxide at the equivalence point of the titration.
Part (c): Calculating the Molar Concentration of NaOH
To find the concentration of the unknown NaOH solution, we must first identify the equivalence point of the titration. The equivalence point is where the moles of the added base (NaOH) are stoichiometrically equal to the initial moles of the acid (HC₂H₃O₂). On a titration curve, this point is located at the center of the steepest, near vertical section of the graph.
By examining the provided data table and graph, we can locate this point. There is a very large jump in pH between the addition of 12.00 mL of NaOH (pH = 5.55) and 14.00 mL of NaOH (pH = 9.35). The inflection point of the curve, representing the equivalence point, is at approximately 14.00 mL of added NaOH.
At this equivalence point:
Moles of NaOH = Moles of HC₂H₃O₂ = 0.0200 mol
We now know the moles of NaOH (0.0200 mol) and the volume of NaOH (14.00 mL) required to reach this point. We can calculate the molarity of the NaOH solution. First, convert the volume to liters:
Volume of NaOH = 14.00 mL = 0.01400 L
Next, use the molarity formula:
Molarity of NaOH = Moles / Volume = 0.0200 mol / 0.01400 L ≈ 1.42857 M
Finally, we round the answer to the correct number of significant figures. The calculation is limited by the initial volume of the acid (10.0 mL), which has three significant figures. Therefore, the concentration of the NaOH solution is 1.43 M.
