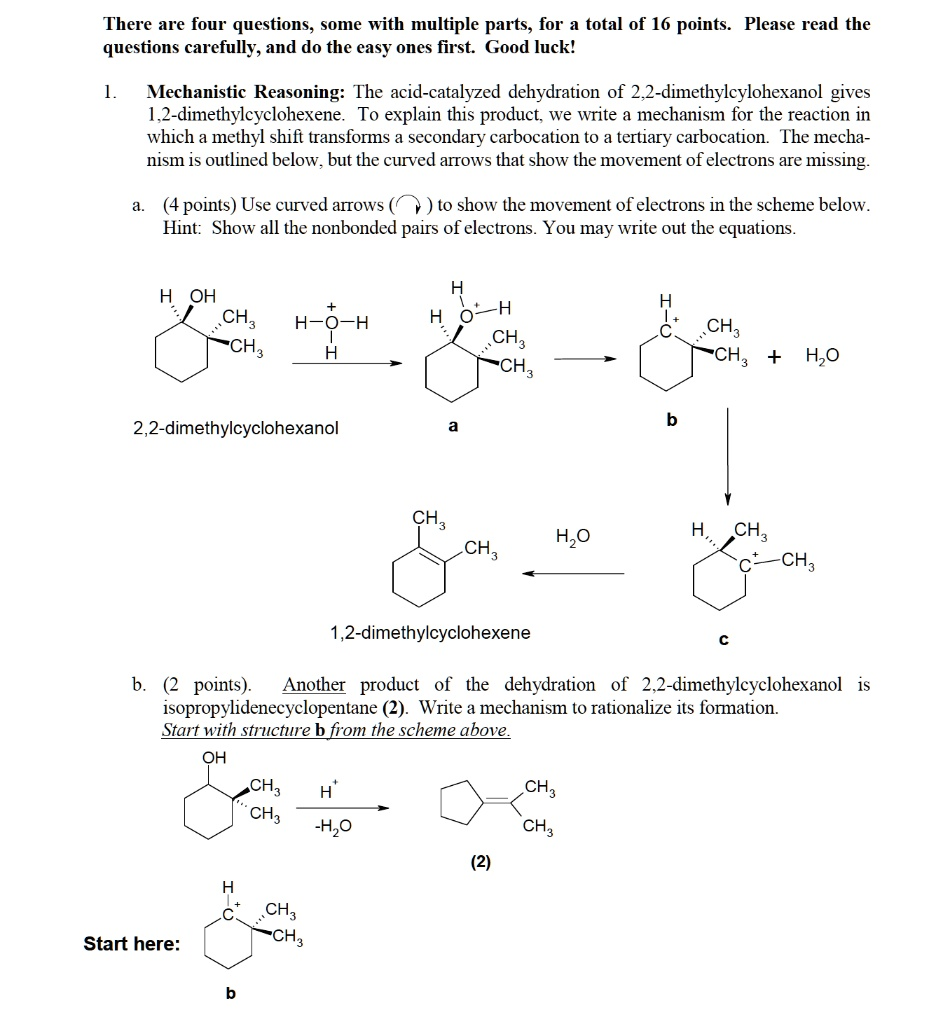
There are four questions, some with multiple parts, for a total of 16 points. Please read the questions carefully, and do the easy ones first. Good luck! 1. Mechanistic Reasoning: The acid-catalyzed dehydration of 2,2-dimethylcylohexanol gives 1,2-dimethylcyclohexene. To explain this product, we write a mechanism for the reaction in which a methyl shift transforms a secondary carbocation to a tertiary carbocation. The mecha- nism is outlined below, but the curved arrows that show the movement of electrons are missing. a. (4 points) Use curved arrows() to show the movement of electrons in the scheme below. Hint: Show all the nonbonded pairs of electrons. You may write out the equations. HOH + CH3 CH3 H-O-H H 2,2-dimethylcyclohexanol H HOH H CCH3 CH3 CH3 CH3 + H?O b a CH3 H?O H, CH CH 3 C-CH3 b. (2 points). 1,2-dimethylcyclohexene C Another product of the dehydration of 2,2-dimethylcyclohexanol is isopropylidenecyclopentane (2). Write a mechanism to rationalize its formation. Start with structure b from the scheme above. OH CH3 H CH3 CH3 -H?O CH3 (2) H C CH3 Start here: CH3 b
The Correct Answer and Explanation is:
