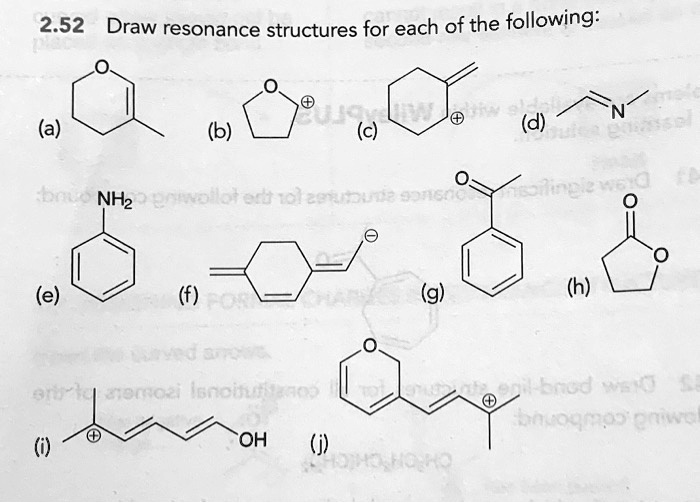
Draw resonance structures for each of the following: (a) (b) (c) 7 NH2 ethanol (e) (g) 0 G8m02i Incih Kod wJ n 07c7 OH
The Correct Answer and Explanation is:
Of course. Here are the resonance structures for each compound, along with a detailed explanation.
Resonance Structures
Here are the resonance structures for each molecule (a) through (j). The original structure is shown first, followed by its resonance contributors. Curved arrows on the original structure indicate the electron movement that leads to the next structure.
(a)
The lone pair on the oxygen atom is adjacent to a pi bond (enol ether). The lone pair can form a double bond with carbon, pushing the pi electrons from the C=C bond onto the adjacent carbon.

(b)
A lone pair on the oxygen atom is adjacent to a carbocation. The lone pair can form a double bond to neutralize the positive charge on the carbon, placing the positive charge on the oxygen. This second structure is a major contributor because all atoms have a full octet.

(c)
This is an allylic carbocation, where a positive charge is adjacent to a C=C double bond. The pi electrons can move toward the carbocation, shifting the double bond and relocating the positive charge.

(d)
In an imine, the pi electrons of the C=N double bond can move onto the more electronegative nitrogen atom, creating a charge-separated structure with a positive charge on carbon and a negative charge on nitrogen.

(e)
In aniline, the lone pair on the nitrogen is adjacent to the benzene ring’s pi system. The lone pair can delocalize into the ring, creating a negative charge that is shared by the ortho and para positions.

(f)
This molecule contains a conjugated system of pi bonds. Electrons can be moved across the system to generate a zwitterionic (charge-separated) resonance structure that features an aromatic ring.

(g)
In acetophenone, the carbonyl group is an electron-withdrawing group attached to a benzene ring. The pi electrons from the C=O bond can move to the oxygen, and the resulting positive charge on the carbonyl carbon can be delocalized into the benzene ring at the ortho and para positions.

(h)
This lactone (cyclic ester) has two important resonance patterns. First, the carbonyl pi electrons can move to the oxygen. Second, the lone pair on the other oxygen atom in the ring can delocalize to form a double bond with the carbonyl carbon.

(i)
This is a delocalized carbocation. The positive charge is stabilized by conjugation with the two double bonds and the lone pairs on the terminal oxygen atom. The charge is distributed across multiple atoms, with a particularly stable contributor where oxygen bears the positive charge and all atoms have an octet.

(j)
This large, conjugated system allows for extensive delocalization of the positive charge. The charge can move along the carbon chain and into the dihydropyran ring, ultimately being stabilized by the ring’s oxygen atom.

