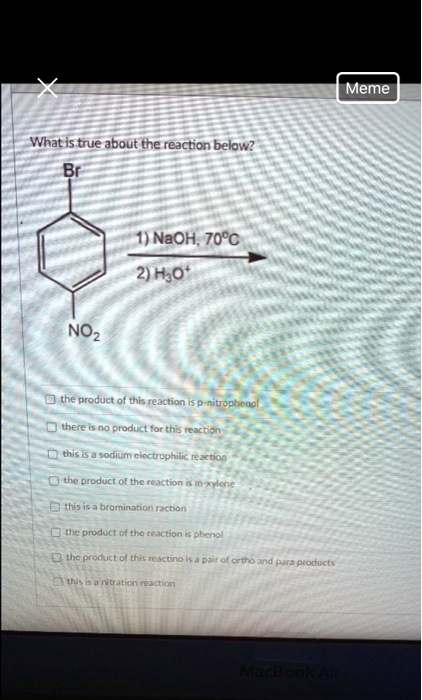
What is true about the reaction below? 1) NaOH; 70°C 2) H2O The product of this reaction is: – None of the above There is no product for this reaction. This is a sodium electrophilic reaction. Produced tetrahydrofuran. None of the above. This is bromination reaction. The product is: The reaction is an elimination reaction. None of the above. Dichloromethane. Ethanol. 3 NO2
The Correct Answer and Explanation is:
The correct statement is: the product of this reaction is p-nitrophenol
Explanation:
The reaction shown is a classic example of a Nucleophilic Aromatic Substitution (SNAr) reaction. This type of reaction involves a nucleophile replacing a leaving group on an aromatic ring.
Reactants and Conditions:
- Starting Material: The molecule is 1-bromo-4-nitrobenzene. This is an aryl halide with a bromine atom (a good leaving group) attached to a benzene ring.
- Activating Group: Critically, there is a nitro group (NO₂) in the para position relative to the bromine. The nitro group is a very strong electron-withdrawing group.
- Reagents:
- NaOH, 70°C: Sodium hydroxide provides the hydroxide ion (OH⁻), which acts as a strong nucleophile. The heat (70°C) provides the necessary activation energy for the reaction to proceed.
- H₃O⁺: This is a source of acid, used in a final “workup” step.
Reaction Mechanism:
Normally, benzene rings are resistant to nucleophilic attack because of their high electron density. However, the presence of the strong electron-withdrawing nitro group at the para position makes the ring “electron-poor” and susceptible to attack by a nucleophile.
- Nucleophilic Attack: The hydroxide ion (OH⁻) attacks the carbon atom bonded to the bromine. This forms a negatively charged intermediate called a Meisenheimer complex. The electron-withdrawing nitro group helps to stabilize this intermediate by delocalizing the negative charge through resonance, pulling the charge out of the ring and onto its own oxygen atoms. This stabilization is key to allowing the reaction to occur.
- Formation of Phenoxide: The leaving group, bromide (Br⁻), departs, and the aromaticity of the ring is restored. This forms sodium p-nitrophenoxide.
- Protonation: The addition of acid (H₃O⁺) in the second step protonates the negatively charged oxygen of the phenoxide, forming the final, neutral product: p-nitrophenol.
Therefore, the bromine atom is substituted by a hydroxyl group, resulting in p-nitrophenol. The other options are incorrect as this is a nucleophilic substitution, not bromination, nitration, or an electrophilic reaction, and the product is specifically the substituted p-nitrophenol, not phenol or m-xylene.
