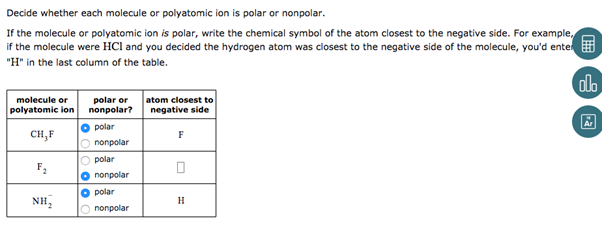
Decide whether each molecule or polyatomic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you’d ente “H” in
The Correct Answer and Explanation is :
To determine if a molecule or polyatomic ion is polar or nonpolar, we need to look at two factors:
- Electronegativity Difference: The electronegativity of atoms within the molecule determines how electrons are distributed. If one atom is more electronegative, it will attract the electrons more strongly, creating a dipole moment (a separation of charge). If the electronegativity difference is significant, the molecule is likely polar.
- Molecular Geometry: Even if a molecule has polar bonds, it can be nonpolar overall if its shape causes the dipoles to cancel out. For example, in a linear molecule with two atoms of the same element (like CO2), the dipoles cancel each other, making the molecule nonpolar. In contrast, if the shape of the molecule is asymmetrical (such as H2O), the dipoles do not cancel, making it polar.
Example of Polar Molecules:
Water (H₂O):
- Electronegativity: Oxygen (3.44) is more electronegative than hydrogen (2.20), so the oxygen atom pulls electrons toward it, creating a dipole.
- Molecular Geometry: Water has a bent shape, so the dipoles do not cancel. Therefore, water is polar with oxygen being closer to the negative side.
Ammonia (NH₃):
- Electronegativity: Nitrogen (3.04) is more electronegative than hydrogen (2.20).
- Molecular Geometry: Ammonia has a trigonal pyramidal shape, so the dipoles do not cancel. Nitrogen is closer to the negative side, making ammonia polar.
Example of Nonpolar Molecules:
Carbon Dioxide (CO₂):
- Electronegativity: Oxygen (3.44) is more electronegative than carbon (2.55), creating polar bonds.
- Molecular Geometry: However, CO₂ has a linear shape, and the two dipoles (one from each C=O bond) cancel each other out. Therefore, CO₂ is nonpolar.
Methane (CH₄):
- Electronegativity: Carbon (2.55) and hydrogen (2.20) have a small electronegativity difference, so the C-H bonds are weakly polar.
- Molecular Geometry: Methane has a tetrahedral shape, so the dipoles cancel out. Therefore, methane is nonpolar.
Summary:
- Polar molecules have a significant electronegativity difference and an asymmetrical shape.
- Nonpolar molecules may have polar bonds but a symmetrical shape or a minimal electronegativity difference between atoms.
If you have specific molecules or ions to analyze, I can walk through them individually as well!