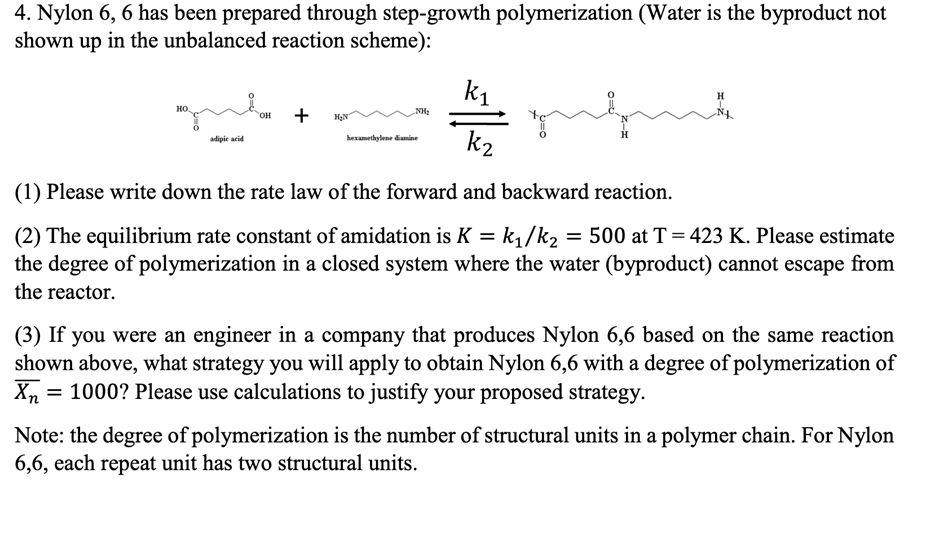
Nylon 6, 6 has been prepared through step-growth polymerization (Water is the byproduct not in the unbalanced reaction scheme): shown up ki H O=D ??, OH + NH2 HON NA adipic acid hexamethylene diamine k2 (1) Please write down the rate law of the forward and backward reaction. = (2) The equilibrium rate constant of amidation is K = ki/k2 = 500 at T = 423 K. Please estimate the degree of polymerization in a closed system where the water (byproduct) cannot escape from the reactor. (3) If you were an engineer in a company that produces Nylon 6,6 based on the same reaction shown above, what strategy you will apply to obtain Nylon 6,6 with a degree of polymerization of X = 1000? Please use calculations to justify your proposed strategy. = Note: the degree of polymerization is the number of structural units in a polymer chain. For Nylon 6,6, each repeat unit has two structural units.
The Correct Answer and Explanation is :
The production of Nylon 6,6 involves the step-growth polymerization between adipic acid and hexamethylene diamine. The unbalanced reaction involves the formation of Nylon 6,6, with water as the byproduct. The forward reaction involves the amidation of the two monomers, and the backward reaction involves the reverse of amidation, where water breaks the bond formed.
1. Rate Law of Forward and Backward Reactions:
For the polymerization of Nylon 6,6:
- Forward Reaction (amidation):
The rate law for the forward reaction can be expressed as:
[
\text{Rate}_{\text{forward}} = k_1 [\text{adipic acid}] [\text{hexamethylene diamine}]
]
where (k_1) is the rate constant for the forward reaction. - Backward Reaction (hydrolysis):
The rate law for the backward reaction (hydrolysis of the polymer) is:
[
\text{Rate}_{\text{backward}} = k_2 [\text{Nylon 6,6}] [\text{water}]
]
where (k_2) is the rate constant for the backward reaction.
2. Estimation of Degree of Polymerization:
The equilibrium rate constant (K) is given by the ratio of the forward rate constant to the backward rate constant:
[
K = \frac{k_1}{k_2} = 500 \quad \text{at} \quad T = 423 \, \text{K}
]
In a closed system, the water byproduct cannot escape, so the degree of polymerization (DP) is influenced by the equilibrium conversion. For step-growth polymerization, the degree of polymerization is related to the monomer conversion (X) by the equation:
[
DP = \frac{1}{1 – X}
]
where (X) is the fraction of monomer converted into polymer.
The equilibrium conversion (X_{\text{eq}}) is determined by the equilibrium constant (K). For this reaction:
[
K = \frac{[\text{Polymer}] [\text{water}]}{[\text{monomers}]} \quad \text{and} \quad X = \frac{[\text{monomer initial}] – [\text{monomer at equilibrium}]}{[\text{monomer initial}]}
]
Since the equilibrium conversion is typically small for high-molecular weight polymers, the degree of polymerization in a closed system will be finite and directly related to the extent of the forward reaction.
3. Strategy for Achieving a DP of 1000:
To obtain a degree of polymerization of (DP = 1000), we need to achieve a high conversion of the monomers. Given that the degree of polymerization (DP = 1000), the conversion (X) should be:
[
X = 1 – \frac{1}{1000} = 0.999
]
Since the degree of polymerization is inversely related to the conversion, a conversion of nearly 100% is required.
However, achieving such high conversion in practice is challenging due to the equilibrium of the reaction, which prevents the complete conversion of monomers into polymer. To overcome this, strategies such as:
- Removal of water: By removing water as it forms, you can shift the equilibrium toward polymer formation.
- Using excess monomer: By adding excess adipic acid or hexamethylene diamine, you can drive the reaction toward more polymerization.
In a real-world setting, engineers would design a reactor system that allows for the removal of water continuously to shift the equilibrium toward the formation of Nylon 6,6 and maximize the polymerization rate. Additionally, adjusting the temperature and pressure could further favor the forward reaction, increasing the extent of polymerization and achieving the desired DP.