Choose one reagent from the Table to carry out the following conversions. Use letters from the table to specify reagents. Reagents O3 d KMNO4 / H3O* 1. Br2 2. KOH in ethanol a g b H2 / Lindlar catalyst h H2O / H2SO4, HgSO4 1. NANH2 in NH3 2. CH�·Br e i 1. BH3 in THF 2. H2O2 in NaOH 1 equiv Br2 Li in NH3 Visited �°) CH3-CH2-C=CH CH3-CH2-C-CH3 Br b) C=CH Br
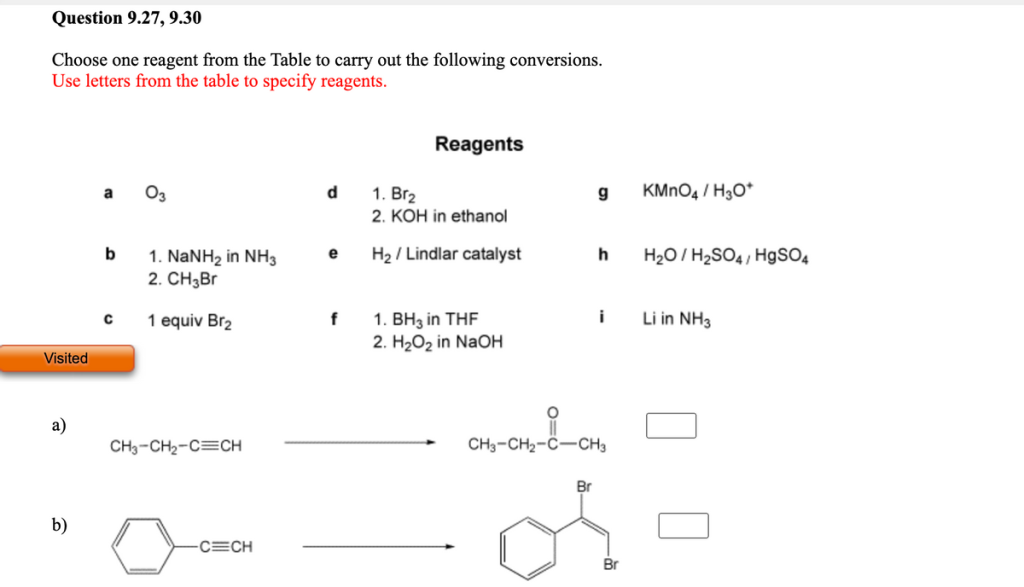
Choose one reagent from the Table to carry out the following conversions. Use letters from the table to specify reagents. Visited a) b) a b C 03 1. NaNH2 in NH3 2. CH3Br 1 equiv Brâ‚‚ CH3-CH2-C=CH -C=CH d e f Reagents 1. Brâ‚‚ 2. KOH in ethanol Hâ‚‚ / Lindlar catalyst 1. BH3 in THF 2. Hâ‚‚Oâ‚‚ in NaOH g KMnO4/H3O* Br h Hâ‚‚O/Hâ‚‚SO4/ HgSO4 i O CH3-CHâ‚‚-C-CH3 Br Li in NH3
The Correct Answer and Explanation is :
The question presents a series of conversions involving reagents, with the task of selecting the appropriate one for each transformation. Let’s break down the two conversions and how the reagents in the provided table apply.
a) CH3-CH2-C=CH → CH3-CH2-C-CH3 (Conversion of an alkene to an alkane)
In this case, the reagent needed is hydrogen (H2) with Lindlar catalyst (reagent b). This process involves a partial hydrogenation reaction where the Lindlar catalyst is used to hydrogenate the alkyne to an alkene, but it does not reduce the alkyne all the way to an alkane. The Lindlar catalyst specifically gives a cis-alkene product. However, since the desired product is an alkane (CH3-CH2-C-CH3), further hydrogenation with H2 and Lindlar’s catalyst can be used to complete the conversion.
b) C=CH Br → CH3-CH=CH2 (Conversion of an alkene to a halide)
Here, the reagent needed is H2O/H2SO4 with HgSO4 (reagent h). This reaction is an example of a hydration of an alkyne. In the presence of sulfuric acid and mercury sulfate, the alkyne undergoes an electrophilic addition reaction. The mercuric ion (Hg2+) catalyzes the addition of water to the alkyne, resulting in the formation of a ketone or aldehyde depending on the structure. Since this step leads to an intermediate product with the desired structure, you can end up with an alkene (CH3-CH=CH2).
Explanation:
- Lindlar catalyst: This is used for selective hydrogenation of alkynes to cis-alkenes and is essential when you want to control the reduction step, avoiding a complete saturation of the molecule to an alkane. It’s used in reactions where partial reduction is desired.
- Hydration (H2O/H2SO4/HgSO4): This is a common method for hydrating alkynes to form carbonyl compounds, and it often leads to the Markovnikov addition, meaning the hydrogen adds to the carbon with more hydrogens, and the -OH (or -O group) adds to the carbon with fewer hydrogens. This gives rise to the formation of enol intermediates that quickly tautomerize into ketones or aldehydes.
Thus, the correct reagents for the conversions are b) H2/Lindlar catalyst and h) H2O/H2SO4/HgSO4.