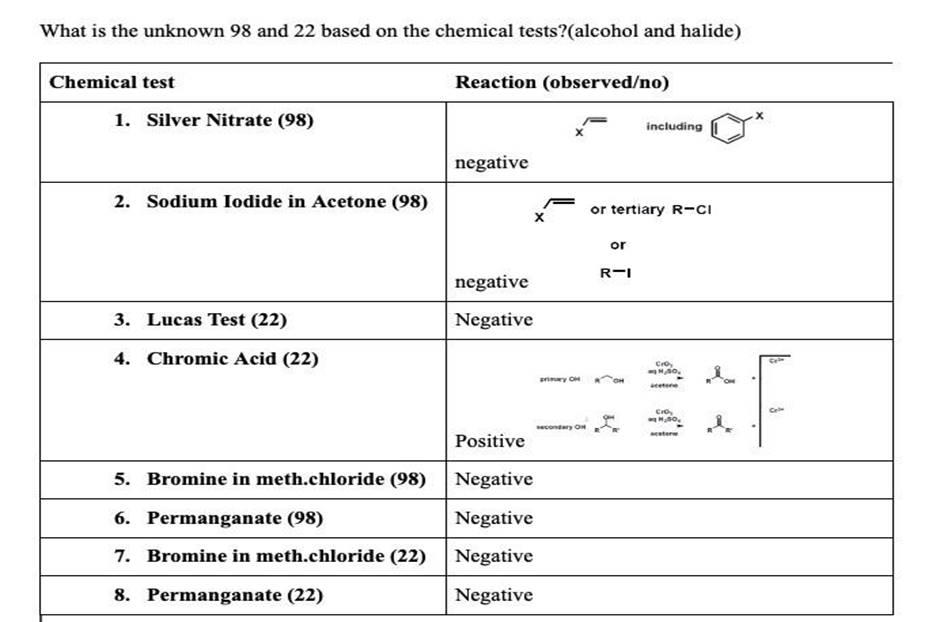
What is the unknown 98 and 22 based on the chemical tests?(alcohol and halide) Chemical test Reaction (observed/no) X 1. Silver Nitrate (98) including ? negative 2. Sodium Iodide in Acetone (98) ? or tertiary R-CI or R- negative 3. Lucas Test (22) Negative 4. Chromic Acid (22) Cro H., PON AH CIO H.10 econdary OH Positive 5. Bromine in meth.chloride (98) Negative 6. Permanganate (98) Negative 7. Bromine in meth.chloride (22) Negative 8. Permanganate (22) ) Negative 1.548 BESEN 13981 1373 SSET CVET LEFT 1-319 1.302 10-905 0.88 -0.870 C.10.53 t: 13.4 | 22 BE 13.4 312 3.0 28 2.6 2.4 22 0 16 10 pp 0.126 66610 0.659 3.2 3.256 3.238 3.220 3.0 2.8 H: 5.60 (: 30,32 26 2.4 2.2 2.0 0.667 1.918 1.900 1.882 -1.864 1.846 1.8 1.6 98 1.29 1.4 1.448 P1.441 1.432 1.427 1.421 1.412 1.410 1.403 -1.396 1.392 1.379 11.362 1.2 1.0 1.000 -0.979 0.962 0.957 -0.944 ppm Sample Information Analysed by Analyse Simple Type Led 11/10/2015 RO SAM Sample 22 val Sample ID Sample Amount Die Fier 19 Vol Duteile CÁC MSM là ta lai chau le tungle là già 0 Mello COCMS Dhe Dumple ICCCMSeitliamentoral ????? Reptile Tuning Pilie COSTune… Modified by Admin Modul 1015 10:52 AM DF Or Methode Le R.Time Raw Make A 24.37) DIMAGE 100 30 60 30 40 10 30 110 130 10 M. Tohle Link 216) Me Maw Mag 24 31-170 B1804) DO Mdee 112) Ali Ini Hello 35.00 29 3.40 2 119.30 154 100.00 29 Lined: R.Time:4.5(Scunt:296) Raw Mode: Averaged 4.4.4.6287-309) BusPeak:7(107370) BG Mode:Averaged 6.1-9.9(498-945) Group 1 – Eventi 100 90 xo 70 60 50 40 30 20 10 33 50 60 70 90 100 110 120 130 140 150 160 170 180 190 200 210 220 230 240 250 260 270 280 200 Miss Table Linel R.Time.tScnt:296) Raw Mode: Averaged 4.4.4.4287.309) BusPeak: 71(107870) BG Mode: Averaged 6.1.9.94495-948) Group Event Abr. In Rel. In 1 55.05 9050 8.39 2 71.10 107870 100.00 3 72.10 5.99 4 126.00 4283 3.92 5 140.35 2420 6 15485 3707 7 199.00 16225 15.04 98 mo 50 ee 1300 1000 500 22 mo 1000 0.00 1000 300 98
The Correct Answer and Explanation is :
Based on the chemical tests you’ve listed and their observed results, the numbers 98 and 22 likely correspond to two distinct compounds or samples that underwent testing for their reactivity and structural properties, based on the chemical tests performed.
Here’s a breakdown of the tests and possible interpretations:
1. Silver Nitrate (98):
Result: Negative
- This test typically identifies halides (Cl⁻, Br⁻, I⁻) in organic compounds by forming precipitates with silver ions. A negative result indicates that no halide ions (chloride, bromide, or iodide) are present in sample 98.
2. Sodium Iodide in Acetone (98):
Result: Negative
- The sodium iodide test in acetone is used to detect the presence of alkyl halides, particularly tertiary halides. A negative result suggests that sample 98 does not contain a tertiary alkyl halide.
3. Lucas Test (22):
Result: Negative
- The Lucas test is used to distinguish between primary, secondary, and tertiary alcohols. A negative result means that sample 22 is likely a primary alcohol or does not have an alcohol group that reacts in this manner.
4. Chromic Acid (22):
Result: Positive
- Chromic acid is used to test for the presence of primary or secondary alcohols (which get oxidized to aldehydes or ketones, respectively). A positive result indicates that sample 22 is a secondary alcohol or can be oxidized.
5. Bromine in Methanol (98):
Result: Negative
- This test checks for the presence of unsaturation (double bonds). A negative result suggests that sample 98 does not have double bonds or is not an alkene.
6. Permanganate (98):
Result: Negative
- Potassium permanganate is another test for unsaturation. A negative result means that sample 98 does not contain alkenes or other compounds that would be oxidized by permanganate.
7. Bromine in Methanol (22):
Result: Negative
- Similar to the test for sample 98, the negative result for sample 22 indicates no unsaturation or alkenes.
8. Permanganate (22):
Result: Negative
- Again, a negative result here suggests the absence of unsaturation in sample 22.
Interpretation:
- Sample 98: The negative results in the tests for halides, tertiary alkyl groups, and unsaturation suggest that this sample could be a saturated alcohol (possibly a primary or secondary alcohol) or a compound that does not contain reactive halide or double bonds.
- Sample 22: The positive result in the chromic acid test suggests it is likely a secondary alcohol, as secondary alcohols are oxidized by chromic acid, whereas primary alcohols are oxidized to aldehydes.
Conclusion:
- Sample 98 likely represents a saturated alcohol or another non-reactive compound, whereas sample 22 appears to be a secondary alcohol based on the positive reaction with chromic acid. The tests suggest no halides or alkenes are present in either sample.
This analysis suggests that the unknown compounds could be a saturated alcohol (primary or secondary) for sample 98, and a secondary alcohol for sample 22. The chemical reactions conducted provide insights into the functional groups present in each sample and their reactivity.