Draw the Lewis Structure for NaOH. Fill octets with lone pairelectrons. Assign a formal charge if available.I’m new to Organic Chemistry. This is what I got sofar.1. Total valence electrons = 1 + 6 + 1 = 8 e-
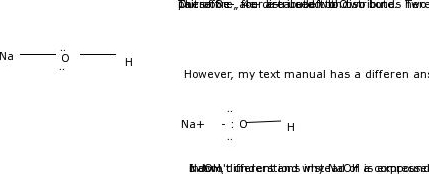
The Correct Answer and Explanation is :
To draw the Lewis structure of NaOH, we need to account for the total valence electrons and ensure proper bonding while satisfying the octet rule (except for hydrogen, which can have only 2 electrons).
Step-by-Step Explanation:
- Determine the total number of valence electrons:
- Sodium (Na): Sodium is in group 1, so it has 1 valence electron.
- Oxygen (O): Oxygen is in group 16, so it has 6 valence electrons.
- Hydrogen (H): Hydrogen is in group 1, so it has 1 valence electron. Therefore, the total number of valence electrons is:
[
1 (\text{Na}) + 6 (\text{O}) + 1 (\text{H}) = 8 \, \text{electrons}
]
- Determine the central atom:
Oxygen (O) is the most electronegative element and will be the central atom, with hydrogen (H) attached to it and sodium (Na) ionically bonded. - Draw the skeleton structure:
The oxygen (O) atom will be bonded to both hydrogen (H) and sodium (Na). Sodium, being a metal, will lose its single valence electron to form a Na⁺ ion, which is electrostatically attracted to the hydroxide (OH) group. - Distribute electrons:
- The bonding between Na and O is ionic, so there is no shared electron pair between them. Na⁺ has no electrons left.
- Oxygen will form a single bond with hydrogen (H), sharing one pair of electrons (2 electrons).
- Oxygen will need two lone pairs to complete its octet, as it initially has 6 electrons, and it shares 2 with hydrogen.
- Place lone pairs:
Oxygen will have two lone pairs (4 electrons) to complete its octet. The hydrogen atom has a duet (2 electrons) from the single bond with oxygen. - Formal charges:
- Sodium (Na): Sodium has lost its 1 valence electron, so it has a formal charge of +1.
- Oxygen (O): Oxygen shares 2 electrons with hydrogen and has 4 electrons as lone pairs. Its formal charge is 0 because it has 6 valence electrons (2 from the bond and 4 from lone pairs), which matches its group number (6).
- Hydrogen (H): Hydrogen shares 1 electron with oxygen, so it has no formal charge (0).
Final Lewis Structure:
H - O⁻
|
Na⁺Explanation:
- Sodium (Na) donates its electron to the oxygen atom, forming an Na⁺ ion.
- Oxygen, now negatively charged (O⁻), forms a bond with hydrogen (H) by sharing a pair of electrons.
- Oxygen satisfies the octet rule with two lone pairs, and hydrogen satisfies the duet rule with the single bond.
This structure shows a hydroxide ion (OH⁻) bonded to a sodium ion, forming sodium hydroxide (NaOH). The formal charges are correctly assigned: Na⁺, O⁻, and H with no charge.
Feel free to ask if you need further clarification on any part of this!