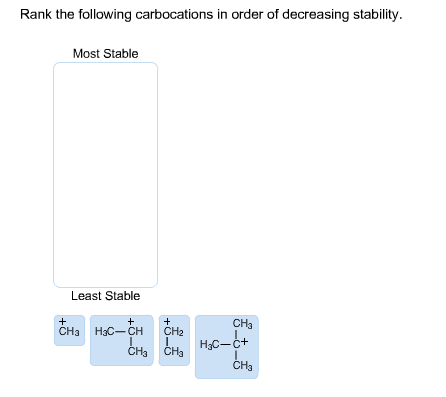
Rank the following carbocations in order of decreasing stability. Most Stable Least Stable Least Stable
The correct answer and explanation is:
To rank the carbocations in order of decreasing stability, it is essential to consider factors such as inductive effects, resonance, and hyperconjugation. Carbocation stability increases as it is more stabilized by these effects.
Let’s break down the typical stability ranking for carbocations:
- Tertiary Carbocation – Most stable: A tertiary carbocation (where the positive charge is on a carbon that is bonded to three other carbon atoms) is the most stable. This stability arises from hyperconjugation and inductive effects. The three alkyl groups around the positively charged carbon provide electron-donating effects, which help to stabilize the electron-deficient species.
- Secondary Carbocation – Moderately stable: A secondary carbocation (where the positively charged carbon is bonded to two other carbon atoms) is less stable than a tertiary carbocation but more stable than a primary one. The positive charge is still somewhat stabilized by hyperconjugation and inductive effects from the two alkyl groups, but these effects are less pronounced than in the tertiary carbocation.
- Primary Carbocation – Less stable: A primary carbocation (where the positively charged carbon is bonded to only one other carbon atom) is significantly less stable. It has only one alkyl group to donate electrons via hyperconjugation and inductive effects, and thus the positive charge is less stabilized.
- Methyl Carbocation – Least stable: A methyl carbocation (where the positively charged carbon is bonded to no other carbons, only hydrogens) is the least stable. It has no alkyl groups to stabilize the positive charge through inductive effects or hyperconjugation, making it highly electron-deficient and very unstable.
Ranking in Order of Decreasing Stability:
- Tertiary carbocation
- Secondary carbocation
- Primary carbocation
- Methyl carbocation – Least stable.
The key factors that determine this ranking are the number of alkyl groups attached to the carbocation (which increase stabilization) and the ability of the structure to distribute or share the positive charge through resonance or hyperconjugation.