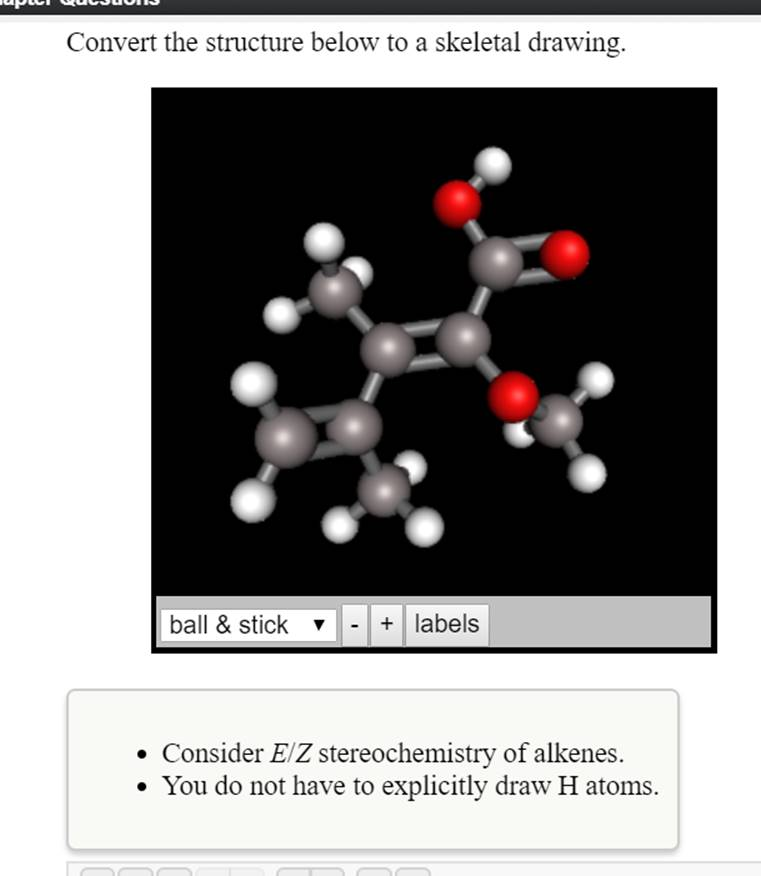
Convert The Structure Below To A Skeletal Drawing. Consider E/Z Stereochemistry Of Alkenes.
The Correct Answer and Explanation is :
To convert a given molecular structure to a skeletal drawing while considering the E/Z stereochemistry of alkenes, follow these steps:
- Identify the Alkene Functional Group: Locate the double bond (C=C) within the molecule.
- Assign Priorities to Substituents: For each carbon of the double bond, assign priorities to the attached substituents using the Cahn-Ingold-Prelog priority rules. This involves comparing the atomic numbers of atoms directly attached to each carbon. The substituent with the higher atomic number receives the higher priority.
- Determine the Stereochemistry:
- (E)-Isomer: If the higher-priority substituents on each carbon are on opposite sides of the double bond, the configuration is (E) (from the German “entgegen,” meaning “opposite”).
- (Z)-Isomer: If the higher-priority substituents are on the same side, the configuration is (Z) (from the German “zusammen,” meaning “together”).
- Construct the Skeletal Structure: In skeletal drawings, carbon atoms are implied at the ends of lines and at intersections. Hydrogen atoms are also implied to satisfy the carbon’s valency of four. Explicitly draw the double bond and indicate the positions of the higher-priority substituents based on the determined stereochemistry.
Example:
Consider 2-butene, which has the molecular formula C₄H₈. The double bond is between the second and third carbon atoms.
- Assign Priorities:
- For the second carbon: the methyl group (–CH₃) has a higher priority than the hydrogen atom.
- For the third carbon: the ethyl group (–C₂H₅) has a higher priority than the hydrogen atom.
- Determine Stereochemistry:
- If the methyl group and ethyl group are on opposite sides of the double bond, the configuration is (E)-2-butene.
- If they are on the same side, the configuration is (Z)-2-butene.
- Skeletal Drawing:
- For (E)-2-butene: draw a line representing the double bond between the second and third carbon atoms, with the methyl group on the left and the ethyl group on the right.
- For (Z)-2-butene: draw the same double bond, but place both the methyl and ethyl groups on the same side.
Understanding E/Z stereochemistry is crucial for accurately representing and predicting the properties and reactivity of alkenes. The E/Z system provides a clear and systematic way to describe the spatial arrangement of substituents around a double bond, which is essential for understanding molecular behavior in chemical reactions.