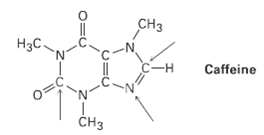
Complete the electron-dot structure of caffeine, showing all lone-pair electrons, and identify the hybridization of the indicatedatoms.
The Correct Answer and Explanation is :
Caffeine (C₈H₁₀N₄O₂) is a stimulant found in coffee, tea, and certain soft drinks. Its structure comprises a central six-membered ring fused to a five-membered ring, both containing nitrogen atoms, along with methyl groups (-CH₃) and carbonyl groups (-C=O).
Electron-Dot Structure:
In the electron-dot structure of caffeine, each atom is represented by its chemical symbol, with dots indicating valence electrons. Lone pairs of electrons are shown as pairs of dots adjacent to the atom. The bonds between atoms are depicted as lines, with single bonds as single lines and double bonds as double lines.
Hybridization of Atoms:
The hybridization of atoms in caffeine can be determined based on their bonding and electron domains:
- Carbon Atoms (C): The carbon atoms in the six-membered ring are sp² hybridized, as they form three sigma bonds and have one pi bond, resulting in a trigonal planar geometry.
- Nitrogen Atoms (N): The nitrogen atoms in the six-membered ring are sp² hybridized, with one lone pair occupying an sp² orbital. The nitrogen atoms in the five-membered ring are sp³ hybridized, with one lone pair occupying an sp³ orbital.
- Oxygen Atoms (O): The oxygen atoms are sp² hybridized, as they form two sigma bonds and have one lone pair, resulting in a bent geometry.
Explanation:
The electron-dot structure of caffeine illustrates the arrangement of atoms and their valence electrons, providing insight into the molecule’s bonding and geometry. The hybridization of atoms explains the observed bond angles and molecular shapes, which are crucial for understanding caffeine’s chemical properties and its interaction with biological systems.
Understanding the electron-dot structure and hybridization of caffeine is essential for comprehending its chemical behavior, including its ability to interact with adenosine receptors in the brain, which contributes to its stimulating effects.