kmarks Profiles Tab Window Help
Home – ?MyJaxState
x||= ?Aktiv Link
Aktiv Chemistry – ?Dimensional
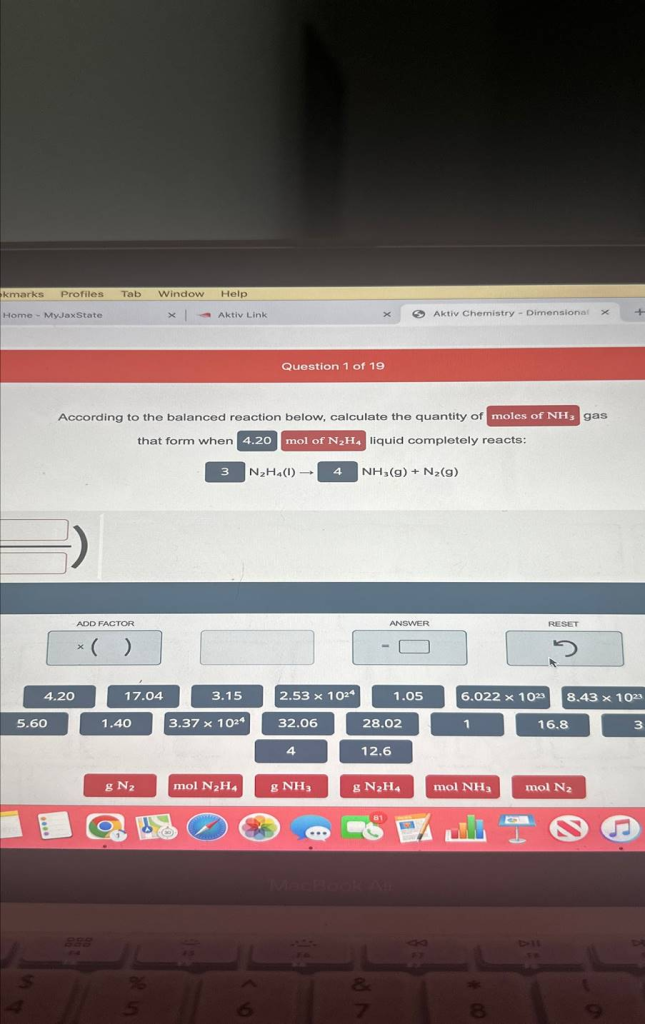
Question 1 ?of 19
According to the balanced reaction below, calculate the quantity of
gas
that form when 4.20mol of N2H4 ?liquid completely reacts:
3N2H4(l)?4,NH3(g)+N2(g)
The correct answer and explanation is:
To calculate the quantity of gas produced when 4.20 mol of N2H4 (liquid) completely reacts, we need to analyze the balanced chemical equation and use stoichiometry.
Step 1: Write the balanced chemical equation
The balanced chemical equation is:
3N2H4(l)→4NH3(g)+N2(g)3N_2H_4(l) \rightarrow 4NH_3(g) + N_2(g)
This tells us that for every 3 moles of N2H4 (liquid), 4 moles of NH3 (gas) and 1 mole of N2 (gas) are produced.
Step 2: Determine the mole ratio
We are given 4.20 mol of N2H4 (liquid), and we need to calculate the total moles of gases produced.
From the balanced equation:
- 3 moles of N2H4 produce 4 moles of NH3 and 1 mole of N2.
The total number of moles of gas produced per 3 moles of N2H4 is: 4 mol NH3+1 mol N2=5 mol of gas4 \, \text{mol NH}_3 + 1 \, \text{mol N}_2 = 5 \, \text{mol of gas}
Step 3: Set up a proportion to calculate the moles of gas produced
If 3 moles of N2H4 produce 5 moles of gas, we can use a simple proportion to calculate the total moles of gas produced by 4.20 mol of N2H4. 3 mol N2H45 mol gas=4.20 mol N2H4x mol gas\frac{3 \, \text{mol N}_2H_4}{5 \, \text{mol gas}} = \frac{4.20 \, \text{mol N}_2H_4}{x \, \text{mol gas}}
Solving for xx: x=5×4.203=7.00 mol of gasx = \frac{5 \times 4.20}{3} = 7.00 \, \text{mol of gas}
Step 4: Conclusion
The total quantity of gas produced when 4.20 mol of N2H4 reacts completely is 7.00 mol. This gas is a combination of both NH3 (ammonia) and N2 (nitrogen gas), as described in the balanced equation. The total molar quantity of gas is simply the sum of the individual gases produced.