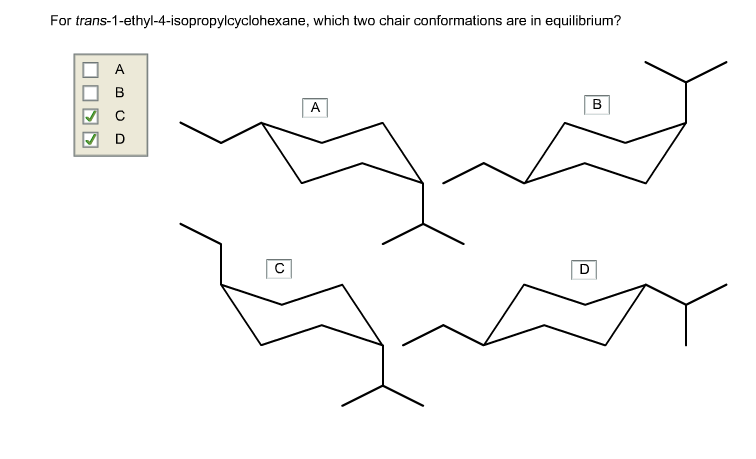
For trans-1-ethyl-4-isopropylcyclohexane, which two chair conformations are in equilibrium?
The Correct Answer and Explanation is :
Trans-1-ethyl-4-isopropylcyclohexane is a disubstituted cyclohexane where the ethyl group is at position 1 and the isopropyl group at position 4. In cyclohexane rings, two chair conformations are possible, each with axial and equatorial positions for substituents. The stability of these conformations depends on the steric interactions between substituents.
Chair Conformations and Substituent Positions:
In the chair conformation, each carbon atom in the cyclohexane ring has one axial and one equatorial position. Axial positions are aligned parallel to the ring’s axis, while equatorial positions extend outward from the ring’s equator. Substituents in axial positions experience 1,3-diaxial interactions, leading to steric strain. Conversely, substituents in equatorial positions are more spatially separated, reducing steric strain and enhancing stability.
Trans-1-Ethyl-4-Isopropylcyclohexane Conformations:
For trans-1-ethyl-4-isopropylcyclohexane, the two chair conformations are:
- Conformation A:
- Ethyl group at position 1: axial
- Isopropyl group at position 4: equatorial
- Conformation B:
- Ethyl group at position 1: equatorial
- Isopropyl group at position 4: axial
Stability Considerations:
In Conformation A, the ethyl group occupies an axial position, leading to 1,3-diaxial interactions with axial hydrogens on carbons 3 and 5. These interactions introduce steric strain, making this conformation less stable.
In Conformation B, the ethyl group is in the more favorable equatorial position, minimizing steric strain. However, the isopropyl group occupies an axial position, resulting in 1,3-diaxial interactions with axial hydrogens on carbons 3 and 5. Despite these interactions, the overall steric strain is lower compared to Conformation A.
Equilibrium and Stability:
The equilibrium between these two conformations favors Conformation B, where the ethyl group is equatorial and the isopropyl group is axial. This preference arises because the steric strain from the axial isopropyl group is less significant than the strain from the axial ethyl group in Conformation A. Therefore, Conformation B is more stable and predominates in equilibrium.
In summary, for trans-1-ethyl-4-isopropylcyclohexane, the two chair conformations in equilibrium are:
- Conformation A: Ethyl axial, isopropyl equatorial
- Conformation B: Ethyl equatorial, isopropyl axial
Conformation B is the more stable of the two and thus predominates in equilibrium.