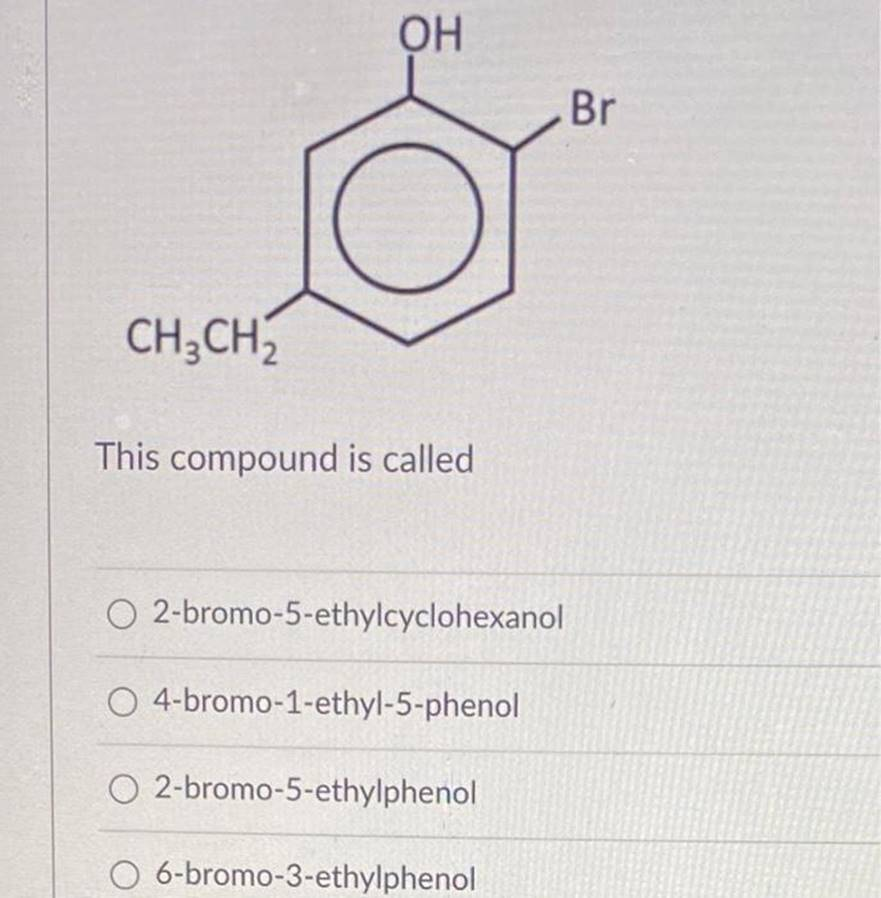
This compound is called 2-bromo-5-ethylcyclohexanol 4-bromo-1-ethyl-5-phenol 2-bromo-5-ethylphenol 6-bromo-3-ethylphenol In a tertiary alcohol, a hydroxzyl group is linked to a(n) disubstituted carbon. triple-bonded carbon. double bonded carbon. trisubstituted carbon

The Correct Answer and Explanation is :
Let’s break down the information in your question.
You are asking about the structure of a tertiary alcohol and a specific compound’s name, followed by identifying the correct type of carbon to which a hydroxyl group is attached.
1. Compound Name:
You provided a list of compounds:
- 2-bromo-5-ethylcyclohexanol
- 4-bromo-1-ethyl-5-phenol
- 2-bromo-5-ethylphenol
- 6-bromo-3-ethylphenol
Based on these names, the hydroxyl group (OH) is likely attached to a carbon in the structure of the compound. The first compound, 2-bromo-5-ethylcyclohexanol, includes the word “cyclohexanol” which indicates the presence of a hydroxyl group attached to a cyclohexane ring.
2. Tertiary Alcohol and Carbon Types:
A tertiary alcohol has a hydroxyl group (-OH) attached to a tertiary carbon, which means the carbon holding the hydroxyl group is connected to three other carbon atoms. In your question, the options you gave were:
- Disubstituted carbon: A carbon connected to two other carbon atoms.
- Triple-bonded carbon: A carbon connected to another carbon by a triple bond.
- Double-bonded carbon: A carbon connected to another carbon by a double bond.
- Trisubstituted carbon: A carbon connected to three other carbon atoms.
The correct answer for the type of carbon that a hydroxyl group is attached to in a tertiary alcohol is trisubstituted carbon, as it is a carbon that is bonded to three other carbon atoms.
Explanation:
In the case of a tertiary alcohol, the hydroxyl group is attached to a tertiary carbon, meaning that the carbon holding the OH group is attached to three other carbon atoms. This is a defining feature of tertiary alcohols. This is in contrast to primary and secondary alcohols, where the hydroxyl group is attached to a primary or secondary carbon, respectively, which are only bonded to one or two other carbon atoms.
In the context of your compounds, if you were referring to a structure such as 2-bromo-5-ethylcyclohexanol, the position of the hydroxyl group would likely be on a carbon within the cyclohexane ring that is bonded to three other carbon atoms, making it a tertiary carbon.