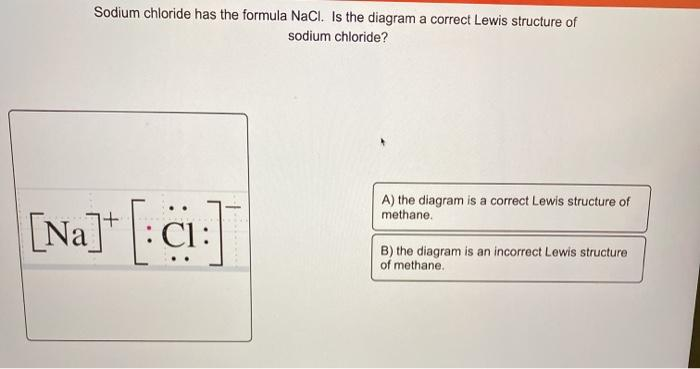
Sodium chloride has the formula NaCl. Is the diagram a correct Lewis structure of sodium chloride? A) the diagram is a correct Lewis structure of methane [Na]+T:01: B) the diagram is an incorrect Lewis structure of methane.
The Correct Answer and Explanation is :
The diagram in question is an incorrect Lewis structure of methane (CH₄). Methane consists of a central carbon atom bonded to four hydrogen atoms, with no lone pairs on the carbon atom. Each hydrogen atom shares one electron with the carbon atom, resulting in four single covalent bonds. This configuration satisfies the octet rule for carbon and the duet rule for hydrogen.
In contrast, sodium chloride (NaCl) is an ionic compound formed by the transfer of an electron from a sodium atom to a chlorine atom. This results in a sodium cation (Na⁺) and a chloride anion (Cl⁻), which are held together by electrostatic forces in a crystal lattice. Since NaCl is an ionic compound, it does not have a traditional Lewis structure. Instead, its structure is better represented by the arrangement of ions in the crystal lattice.
Therefore, the correct answer is B) the diagram is an incorrect Lewis structure of methane.