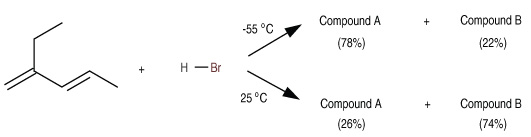
Kinetic vs Thermodynamic Control of an Electrophilic Addition ReactionA student performs the reaction of 2-ethyl-1,3-pentadiene with HBr at –55°C and obtains the following mixture of products: 78% compound A and 22% compound B. She repeats the reaction the next day, and this time obtains the approximately reverse ratio of products: 26% compound A and 74% compound B. Puzzled, she reviews the procedure and realizes that she forgot to regulate the reaction temperature and that the reaction instead proceeded at room temperature (25°C).
Determine the structures of compounds A and B and elucidate the role of temperature in the reaction
BGive the structure of compound A, the major product when the reaction is conducted at low temperatures
The Correct Answer and Explanation is :
In the electrophilic addition of HBr to 2-ethyl-1,3-pentadiene, the reaction can yield two major products:
- Compound A: 3-bromo-2-ethylpentane
- Compound B: 1-bromo-2-ethyl-2-pentene
The distribution of these products is influenced by the reaction temperature, which determines whether the reaction is under kinetic or thermodynamic control.
Kinetic Control at Low Temperature (-55°C):
At low temperatures, reactions are typically irreversible, and the product distribution is governed by the relative rates of formation. The major product is the one that forms fastest, known as the kinetic product. In this case, the addition of HBr to 2-ethyl-1,3-pentadiene proceeds through the formation of a more stable carbocation intermediate, leading predominantly to Compound A (3-bromo-2-ethylpentane) with a 78% yield. This product results from 1,2-addition, where the hydrogen adds to the first carbon of the diene, and the bromine adds to the adjacent carbon. The 1,2-addition pathway has a lower activation energy, making it the kinetically favored product.
Thermodynamic Control at Room Temperature (25°C):
At higher temperatures, reactions can become reversible, allowing the system to reach equilibrium. Under these conditions, the product distribution is determined by the relative stabilities of the products, favoring the thermodynamically more stable product. In this scenario, Compound B (1-bromo-2-ethyl-2-pentene) becomes predominant with a 74% yield. This product arises from 1,4-addition, where the hydrogen adds to the first carbon, and the bromine adds to the fourth carbon of the diene system. The resulting product has a more substituted (and thus more stable) double bond, making it the thermodynamically favored product.
Role of Temperature:
Temperature plays a crucial role in determining the product distribution in electrophilic addition reactions involving conjugated dienes:
- Low Temperature: Favors kinetic control, leading to the major formation of the product that forms fastest (Compound A).
- High Temperature: Favors thermodynamic control, resulting in the predominant formation of the more stable product (Compound B).
This temperature-dependent behavior is a classic example of kinetic versus thermodynamic control in chemical reactions, where low temperatures favor the formation of products with lower activation energies (kinetic products), and higher temperatures allow the system to equilibrate, favoring the more stable (thermodynamic) products.