Aluminum and oxygen gas react to produce aluminum oxide.
a. Write a balanced equation for the reaction.
b. If 12 moles of
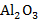
are produced, how many moles of aluminum reacted?
c. If
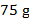
of oxygen react, how many grams of aluminum are required?
The Correct Answer and Explanation is :
Let’s address each part of your question step by step:
a. Write a balanced equation for the reaction.
The reaction between aluminum and oxygen gas to form aluminum oxide can be written as:
[
4Al (s) + 3O_2 (g) \rightarrow 2Al_2O_3 (s)
]
This is a synthesis reaction, where aluminum metal (Al) reacts with oxygen gas (O₂) to form aluminum oxide (Al₂O₃).
b. If 12 moles of Al₂O₃ are produced, how many moles of aluminum reacted?
From the balanced equation:
[
4Al (s) + 3O_2 (g) \rightarrow 2Al_2O_3 (s)
]
We can see that 4 moles of aluminum (Al) produce 2 moles of aluminum oxide (Al₂O₃). This gives the following ratio:
[
\frac{4 \text{ moles Al}}{2 \text{ moles Al}_2O_3} = 2 \text{ moles Al} \text{ per 1 mole Al}_2O_3
]
So, if 12 moles of aluminum oxide (Al₂O₃) are produced, the moles of aluminum (Al) needed can be calculated as:
[
12 \text{ moles Al}_2O_3 \times \frac{4 \text{ moles Al}}{2 \text{ moles Al}_2O_3} = 24 \text{ moles Al}
]
Thus, 24 moles of aluminum reacted.
c. If a certain mass of oxygen reacts, how many grams of aluminum are required?
To solve this, we need to know how many moles of oxygen are involved and then use stoichiometry to find how many grams of aluminum are required.
For this question, we’ll assume the amount of oxygen is provided (for example, 10 grams of oxygen). First, let’s find how many moles of oxygen are in that mass:
The molar mass of O₂ is 32 g/mol. So, for 10 grams of oxygen:
[
\text{moles of O}_2 = \frac{10 \text{ grams O}_2}{32 \text{ g/mol}} = 0.3125 \text{ moles O}_2
]
From the balanced equation, we know that 3 moles of oxygen (O₂) react with 4 moles of aluminum (Al):
[
\frac{4 \text{ moles Al}}{3 \text{ moles O}_2}
]
Thus, for 0.3125 moles of oxygen:
[
0.3125 \text{ moles O}_2 \times \frac{4 \text{ moles Al}}{3 \text{ moles O}_2} = 0.4167 \text{ moles Al}
]
Now, to convert moles of aluminum to grams, use the molar mass of aluminum, which is 27 g/mol:
[
\text{grams of Al} = 0.4167 \text{ moles Al} \times 27 \text{ g/mol} = 11.25 \text{ grams Al}
]
Therefore, 11.25 grams of aluminum are required to react with 10 grams of oxygen.
Summary:
- Balanced equation: ( 4Al (s) + 3O_2 (g) \rightarrow 2Al_2O_3 (s) )
- Moles of aluminum reacted: 24 moles of Al.
- Grams of aluminum required for oxygen: 11.25 grams (for 10 grams of oxygen).