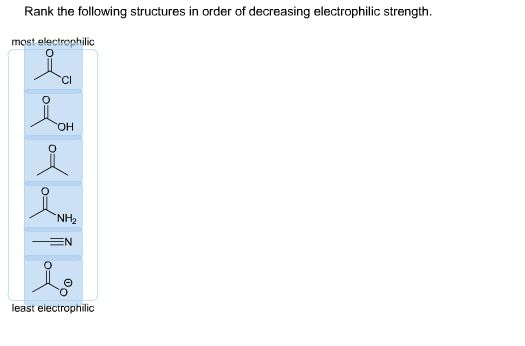
Rank the following structures in order of decreasing electrophile strength. Please explain reasoning if possible.
The correct answer and explanation is:
The electrophilicity of molecules depends on their ability to accept electron density. In general, electrophiles are stronger when they are more electron-deficient or more polarized in a way that makes their electron-accepting site highly positive. The structures in the image appear to include various functional groups, which influence their electrophilic character. Let’s analyze and rank them in terms of decreasing electrophile strength:
Rank (from strongest to weakest electrophile):
- Acid halide (R-CO-Cl)
- Ketone (R-CO-R’)
- Aldehyde (R-CHO)
- Ester (R-COOR’)
- Carboxylic acid (R-COOH)
Explanation:
- Acid halide (R-CO-Cl):
Acid halides are the strongest electrophiles due to the strong electron-withdrawing effect of the halide (Cl). This electronegativity makes the carbonyl carbon highly electron-deficient, enhancing its reactivity toward nucleophiles. - Ketone (R-CO-R’):
In ketones, the carbonyl carbon is polarized, with the oxygen pulling electron density away. The two alkyl groups, however, stabilize the positive charge on the carbonyl carbon through hyperconjugation and inductive effects, making ketones slightly less electrophilic than acid halides. - Aldehyde (R-CHO):
Aldehydes are less electrophilic than acid halides but more so than ketones because they only have one alkyl group to stabilize the carbonyl carbon. This makes the carbonyl carbon more accessible to nucleophiles compared to ketones. - Ester (R-COOR’):
Esters have a lone pair on the oxygen in the -OR group, which donates electron density through resonance to the carbonyl carbon. This reduces the partial positive charge on the carbonyl carbon, decreasing its electrophilicity. - Carboxylic acid (R-COOH):
In carboxylic acids, resonance and hydrogen bonding make the carbonyl carbon less electrophilic. The electron-donating effects of the hydroxyl (-OH) group further reduce the positive charge on the carbonyl carbon.
Summary:
The electrophilicity order is primarily determined by resonance, inductive effects, and the availability of the electron-deficient carbon for nucleophilic attack. Acid halides are the strongest electrophiles due to the electron-withdrawing halogen, while carboxylic acids are the weakest due to resonance stabilization and the electron-donating hydroxyl group.