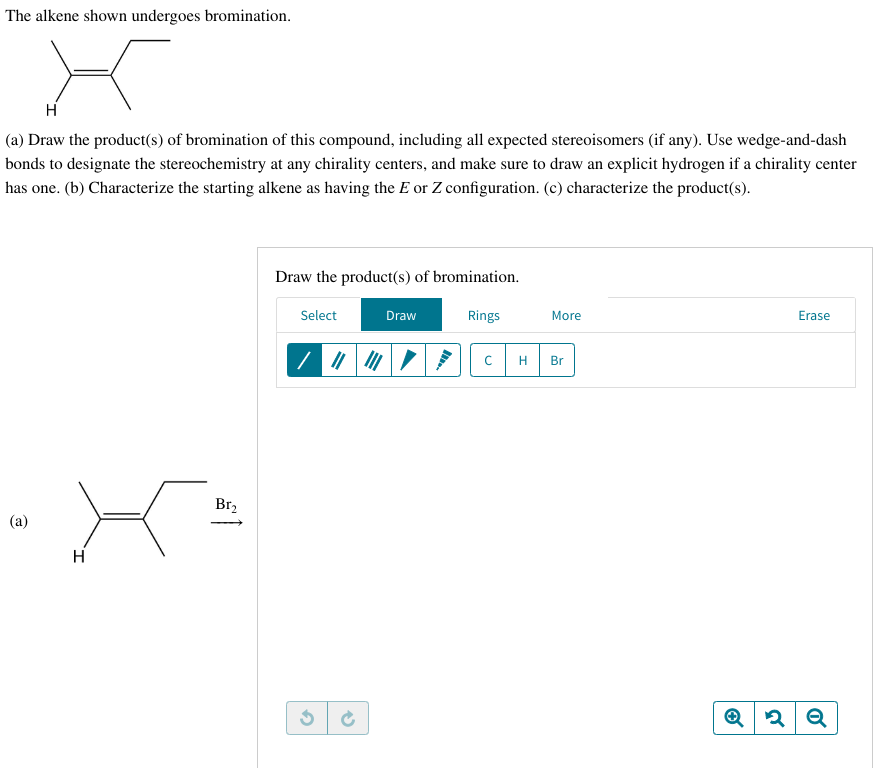
The alkene shown undergoes bromination. (a) Draw the product(s) of bromination of this compound, including all expected stereoisomers (if any). Use wedge‑and‑dash bonds to designate the stereochemistry at any chirality centers, and make sure to draw an explicit hydrogen if a chirality center has one. (b) Characterize the starting alkene as having the E or Z configuration. (c) characterize the product(s).
The alkene shown undergoes bromination.
(a) Draw the product(s) of bromination of this compound, including all expected stereoisomers (if any). Use wedge‑and‑dash bonds to designate the stereochemistry at any chirality centers, and make sure to draw an explicit hydrogen if a chirality center has one. (b) Characterize the starting alkene as having the E or Z configuration. (c) characterize the product(s).
The correct answer and explanation is:
To answer your question thoroughly, I’ll provide a step-by-step explanation. Here’s the solution for the bromination reaction of the given alkene:
Solution
(a) Drawing the Products of Bromination:
- Reaction Mechanism:
Bromination of alkenes occurs via an anti-addition mechanism:- The π bond of the alkene interacts with a bromine molecule (Br₂), forming a bromonium ion intermediate.
- Nucleophilic attack by a bromide ion (Br⁻) opens the bromonium ion, leading to the anti-addition of bromine atoms across the double bond.
- Stereoisomers of the Product:
Since the bromination is an anti-addition, the two bromine atoms will add to opposite faces of the double bond. If the double bond produces chiral centers, stereoisomers will result. - Drawing the Products:
- If the starting alkene has two different groups on each carbon of the double bond, two enantiomers (R,S and S,R configurations) will form due to anti-addition.
(b) Characterizing the Starting Alkene:
- E/Z Configuration:
- The E (entgegen) or Z (zusammen) designation depends on the priority of the groups attached to each carbon of the double bond, according to the Cahn-Ingold-Prelog rules.
- Identify the higher-priority group on each carbon:
- If the higher-priority groups are on the same side of the double bond, the configuration is Z.
- If they are on opposite sides, the configuration is E.
(c) Characterizing the Products:
- Stereochemistry of the Products:
- The products will have two bromine atoms added across the double bond in an anti orientation, resulting in a pair of enantiomers (optically active mixture).
- Explicitly show the wedge and dash bonds for stereochemistry, including the hydrogen at the chiral center.
Explanation (300 words):
Bromination of an alkene is a classic electrophilic addition reaction. The π electrons of the alkene interact with a bromine molecule, forming a cyclic bromonium ion intermediate. This intermediate is highly reactive, and a bromide ion (Br⁻) quickly attacks it from the opposite side of the cyclic structure. The result is an anti-addition of bromine atoms across the double bond.
The stereochemistry of the product depends on the configuration of the starting alkene and the anti-addition mechanism. If the alkene forms chiral centers upon bromination, two enantiomers are generated. The products are represented using wedge-and-dash notation, with wedges indicating bonds coming out of the plane and dashes representing bonds going into the plane.
The starting alkene’s configuration is determined using the E/Z system. This system prioritizes groups attached to the double bond carbons based on atomic numbers. If the high-priority groups are on opposite sides of the double bond, the alkene is E; if on the same side, it is Z.
In the product, the bromines add anti to each other due to steric hindrance in the bromonium ion intermediate. This reaction typically produces racemic mixtures when chiral centers are formed. Understanding these principles is crucial for predicting the stereochemical outcomes of halogenation reactions.
Would you like me to create a diagram for this reaction?To answer your question thoroughly, I’ll provide a step-by-step explanation. Here’s the solution for the bromination reaction of the given alkene:
Solution
(a) Drawing the Products of Bromination:
- Reaction Mechanism:
Bromination of alkenes occurs via an anti-addition mechanism:- The π bond of the alkene interacts with a bromine molecule (Br₂), forming a bromonium ion intermediate.
- Nucleophilic attack by a bromide ion (Br⁻) opens the bromonium ion, leading to the anti-addition of bromine atoms across the double bond.
- Stereoisomers of the Product:
Since the bromination is an anti-addition, the two bromine atoms will add to opposite faces of the double bond. If the double bond produces chiral centers, stereoisomers will result. - Drawing the Products:
- If the starting alkene has two different groups on each carbon of the double bond, two enantiomers (R,S and S,R configurations) will form due to anti-addition.
(b) Characterizing the Starting Alkene:
- E/Z Configuration:
- The E (entgegen) or Z (zusammen) designation depends on the priority of the groups attached to each carbon of the double bond, according to the Cahn-Ingold-Prelog rules.
- Identify the higher-priority group on each carbon:
- If the higher-priority groups are on the same side of the double bond, the configuration is Z.
- If they are on opposite sides, the configuration is E.
(c) Characterizing the Products:
- Stereochemistry of the Products:
- The products will have two bromine atoms added across the double bond in an anti orientation, resulting in a pair of enantiomers (optically active mixture).
- Explicitly show the wedge and dash bonds for stereochemistry, including the hydrogen at the chiral center.
Explanation :
Bromination of an alkene is a classic electrophilic addition reaction. The π electrons of the alkene interact with a bromine molecule, forming a cyclic bromonium ion intermediate. This intermediate is highly reactive, and a bromide ion (Br⁻) quickly attacks it from the opposite side of the cyclic structure. The result is an anti-addition of bromine atoms across the double bond.
The stereochemistry of the product depends on the configuration of the starting alkene and the anti-addition mechanism. If the alkene forms chiral centers upon bromination, two enantiomers are generated. The products are represented using wedge-and-dash notation, with wedges indicating bonds coming out of the plane and dashes representing bonds going into the plane.
The starting alkene’s configuration is determined using the E/Z system. This system prioritizes groups attached to the double bond carbons based on atomic numbers. If the high-priority groups are on opposite sides of the double bond, the alkene is E; if on the same side, it is Z.
In the product, the bromines add anti to each other due to steric hindrance in the bromonium ion intermediate. This reaction typically produces racemic mixtures when chiral centers are formed. Understanding these principles is crucial for predicting the stereochemical outcomes of halogenation reactions.
Would you like me to create a diagram for this reaction?