Calculate the molar mass of phosphoric acid H3PO4.
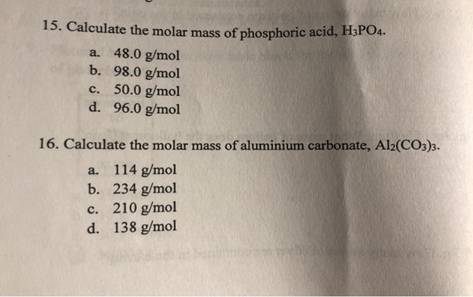

The correct answer and explanation is:
Molar Mass of Phosphoric Acid (H₃PO₄)
The molar mass of phosphoric acid is calculated by summing the atomic masses of its constituent atoms. The chemical formula H3PO4H_3PO_4 indicates it contains:
- Hydrogen (H): 3 atoms
- Phosphorus (P): 1 atom
- Oxygen (O): 4 atoms
Using the periodic table:
- Atomic mass of H = 1.01 g/mol
- Atomic mass of P = 30.97 g/mol
- Atomic mass of O = 16.00 g/mol
Calculation:
Molar mass=(3×1.01)+(1×30.97)+(4×16.00)\text{Molar mass} = (3 \times 1.01) + (1 \times 30.97) + (4 \times 16.00) Molar mass=3.03+30.97+64.00=98.00 g/mol\text{Molar mass} = 3.03 + 30.97 + 64.00 = 98.00 \, \text{g/mol}
Thus, the molar mass of phosphoric acid (H3PO4H_3PO_4) is 98.00 g/mol.
Explanation:
Molar mass is the mass of one mole of a chemical compound, expressed in grams per mole (g/molg/mol). It is a crucial property in chemistry for understanding the quantities of substances involved in chemical reactions.
Steps:
- Understand the Formula: The formula H3PO4H_3PO_4 shows the ratio of atoms in the compound: 3 hydrogens, 1 phosphorus, and 4 oxygens.
- Find Atomic Masses: Atomic masses of individual elements are obtained from the periodic table. These values reflect the average mass of an atom, accounting for isotopes.
- Multiply by Number of Atoms: For each element, multiply its atomic mass by the number of atoms in the compound.
- For hydrogen: 3×1.01 g/mol3 \times 1.01 \, \text{g/mol}
- For phosphorus: 1×30.97 g/mol1 \times 30.97 \, \text{g/mol}
- For oxygen: 4×16.00 g/mol4 \times 16.00 \, \text{g/mol}.
- Sum the Results: Adding these contributions gives the total molar mass.
Phosphoric acid is a triprotic acid widely used in fertilizers, food flavoring, and rust removal. Understanding its molar mass allows chemists to measure precise quantities for reactions, ensuring accurate stoichiometric calculations.