Spectroscopy: Choose the compound that matches the IR spectrum (IV)
Choose the compound responsible for the IR spectrum shown
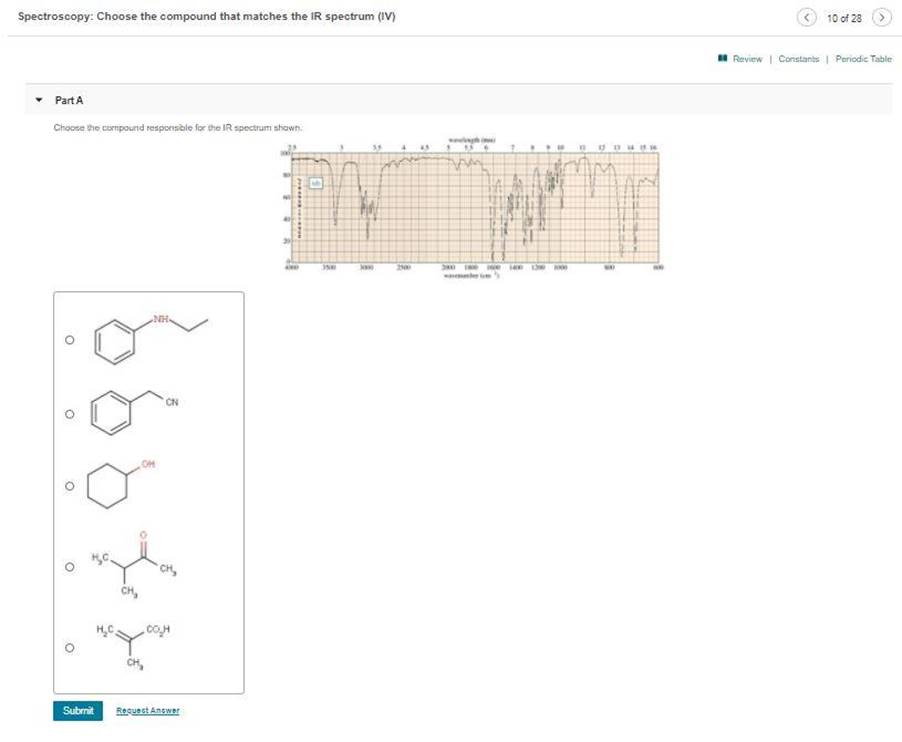
The Correct Answer and Explanation is :
To determine the compound responsible for the given IR spectrum, we analyze the key absorption peaks. The significant absorption bands help identify functional groups and structural features of the molecule. Here’s a step-by-step explanation:
Key Observations from the IR Spectrum
- Broad Peak around 3300 cm⁻¹:
This broad absorption is characteristic of O–H stretching, indicating the presence of an alcohol or phenol group. - No Strong Absorption around 1700 cm⁻¹:
The absence of a sharp, strong peak around this region suggests that a carbonyl group (C=O) is not present in the compound. - C–H Stretching around 2900 cm⁻¹:
Peaks in this region indicate the presence of alkane C–H bonds. - C–O Stretching around 1000–1200 cm⁻¹:
This peak is indicative of the C–O bond in alcohols or ethers.
Likely Compound
Given these observations, the IR spectrum suggests the compound is likely an alcohol. The broad O–H stretch around 3300 cm⁻¹ is a definitive marker for this group, and the absence of a carbonyl group rules out carboxylic acids, esters, ketones, and aldehydes. Additionally, the C–H stretches around 2900 cm⁻¹ and the C–O stretch around 1000–1200 cm⁻¹ align with an alcohol.
Example Compound
A possible match could be ethanol (C₂H₅OH) or a simple primary alcohol. Ethanol shows:
- A broad O–H stretch at ~3300 cm⁻¹.
- Aliphatic C–H stretches around 2900 cm⁻¹.
- C–O stretching in the 1000–1200 cm⁻¹ range.
Conclusion
The compound responsible for this IR spectrum is most likely ethanol or a structurally similar alcohol. This conclusion is based on the absence of carbonyl groups, the presence of a broad O–H stretch, and C–H and C–O stretching consistent with alcohols.