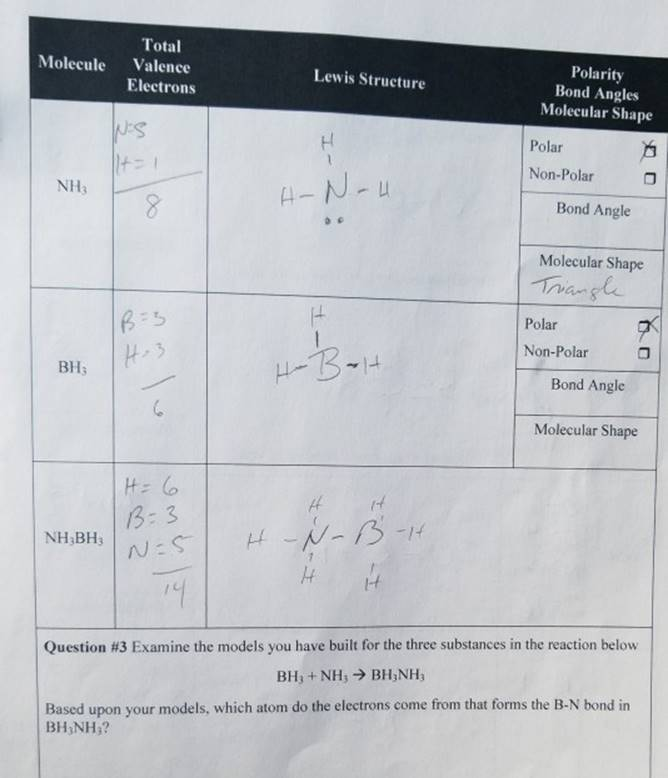
Total Polarity Bond Angles Molecular Shape Molecule Valence Lewis Structure Electrons Polar Non-Polar NHy Al Bond Angle Molecular Shape Triang Polar Non-Polar BH3 Bond Angle Molecular Shape 13 NH,BH3 I-F Question #3 Examine The Models You Have Built For The Three Substances In The Reaction Below Based Upon Your Models, Which Atom Do The Electrons Come From
The Correct Answer and Explanation is :
To accurately address your question, let’s analyze the components based on the molecular models and concepts:
- Ammonia ((NH_3)):
- Bond Angle: Approximately (107^\circ), slightly less than the (109.5^\circ) of a perfect tetrahedral due to lone pair repulsion.
- Molecular Shape: Trigonal pyramidal.
- Polarity: Polar due to its lone pair and the electronegativity of nitrogen.
- Boron Trifluoride ((BH_3)):
- Bond Angle: (120^\circ), characteristic of a trigonal planar structure.
- Molecular Shape: Trigonal planar.
- Polarity: Non-polar because the molecule is symmetric, and the dipoles cancel out.
- Interaction in Reaction ((NH_3) and (BH_3)):
The reaction involves (NH_3) (electron donor) and (BH_3) (electron acceptor). (BH_3) lacks a full octet (boron has only six valence electrons), making it an electrophile. (NH_3) has a lone pair on nitrogen, which can be donated to (BH_3). The product is a coordinate covalent bond between the nitrogen of (NH_3) and the boron of (BH_3), forming a (NH_3BH_3) adduct. - Source of Electrons:
- The electrons come from the lone pair on the nitrogen atom in (NH_3).
- Boron in (BH_3) does not have lone pairs to share; it accepts electrons to complete its octet.
Explanation (300 words):
The reaction between ammonia ((NH_3)) and boron trifluoride ((BH_3)) showcases the principles of molecular geometry, polarity, and electron pair donation. (NH_3) has a trigonal pyramidal shape due to its lone pair of electrons, which repel the bonding pairs, resulting in a bond angle of (107^\circ). Being polar, (NH_3) can interact strongly with other molecules.
In contrast, (BH_3) has a trigonal planar shape and a bond angle of (120^\circ). (BH_3) is non-polar, but boron is electron-deficient, as it does not complete an octet. This makes (BH_3) a strong Lewis acid (electron pair acceptor).
During the reaction, the nitrogen in (NH_3) donates its lone pair of electrons to the electron-deficient boron atom in (BH_3). This forms a coordinate covalent bond, resulting in the adduct (NH_3BH_3). The boron atom accepts the lone pair to achieve an octet configuration, while nitrogen retains its original valence configuration.
In conclusion, the electrons in this reaction come from the lone pair of nitrogen in (NH_3), highlighting nitrogen’s role as a Lewis base. This interaction is fundamental to understanding acid-base chemistry and molecular interactions in reactions involving electron pair transfer.