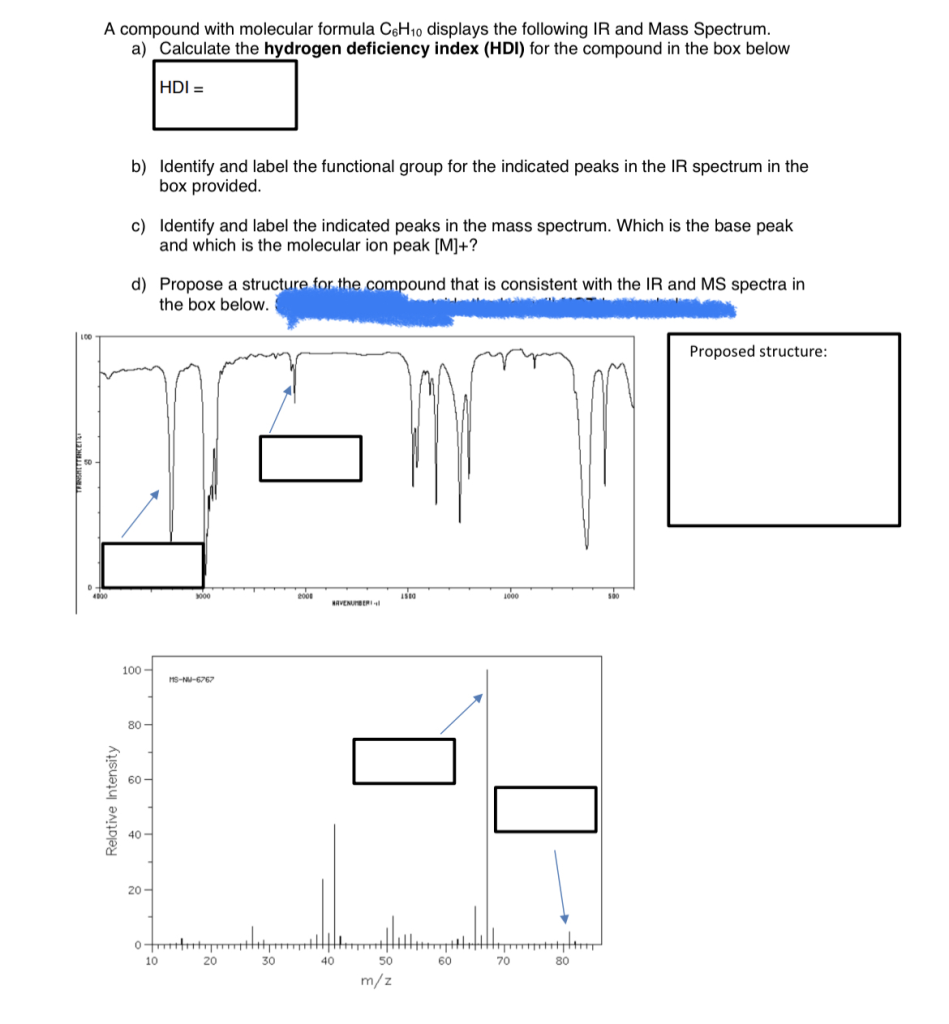
A compound with molecular formula C6H10 displays the following IR and Mass Spectrum. a) Calculate the hydrogen deficiency index (HDI) for the compound in the box below HDI = b) Identify and label the functional group for the indicated peaks in the IR spectrum in the box provided. c) Identify and label the indicated peaks in the mass spectrum. Which is the base peak and which is the molecular ion peak [M]+? d) Propose a structure for the compound that is consistent with the IR and MS spectra in the box below.
The Correct Answer and Explanation is :
To determine the structure of a compound with the molecular formula C₆H₁₀, we can analyze its hydrogen deficiency index (HDI), infrared (IR) spectrum, and mass spectrum.
a) Hydrogen Deficiency Index (HDI):
The HDI indicates the number of degrees of unsaturation (rings and/or multiple bonds) in a molecule. It is calculated using the formula:
[ \text{HDI} = \frac{2C + 2 – H}{2} ]
For C₆H₁₀:
[ \text{HDI} = \frac{2(6) + 2 – 10}{2} = \frac{12 + 2 – 10}{2} = \frac{4}{2} = 2 ]
An HDI of 2 suggests the presence of two degrees of unsaturation, which could be two double bonds, one triple bond, one ring with one double bond, or two rings.
b) IR Spectrum Analysis:
Key absorption peaks in the IR spectrum help identify functional groups:
- ~3000 cm⁻¹: Indicates C–H stretching vibrations.
- ~1650 cm⁻¹: Corresponds to C=C stretching vibrations, characteristic of alkenes.
- ~2100–2260 cm⁻¹: Associated with C≡C stretching vibrations, indicative of alkynes.
The presence of absorption around 1650 cm⁻¹ suggests a C=C bond, while absorption in the 2100–2260 cm⁻¹ range indicates a C≡C bond.
c) Mass Spectrum Analysis:
In the mass spectrum:
- Molecular Ion Peak ([M]⁺): The peak at m/z = 82 corresponds to the molecular ion, confirming the molecular weight of C₆H₁₀.
- Base Peak: The most intense peak, often resulting from a stable fragment.
For cyclohexene, the base peak is observed at m/z = 67, resulting from the loss of a CH₃ group ([M–15]⁺).
d) Proposed Structure:
Considering the HDI of 2 and the spectral data:
- The IR spectrum indicates the presence of both C=C and C≡C bonds.
- The mass spectrum’s molecular ion peak at m/z = 82 aligns with the molecular weight of C₆H₁₀.
A structure consistent with these findings is 1-hexyne, which contains a terminal alkyne group.
In summary, the compound is likely 1-hexyne, as it fits the molecular formula C₆H₁₀, has an HDI of 2, and its IR and mass spectra are consistent with the presence of a triple bond and the observed fragmentation pattern.