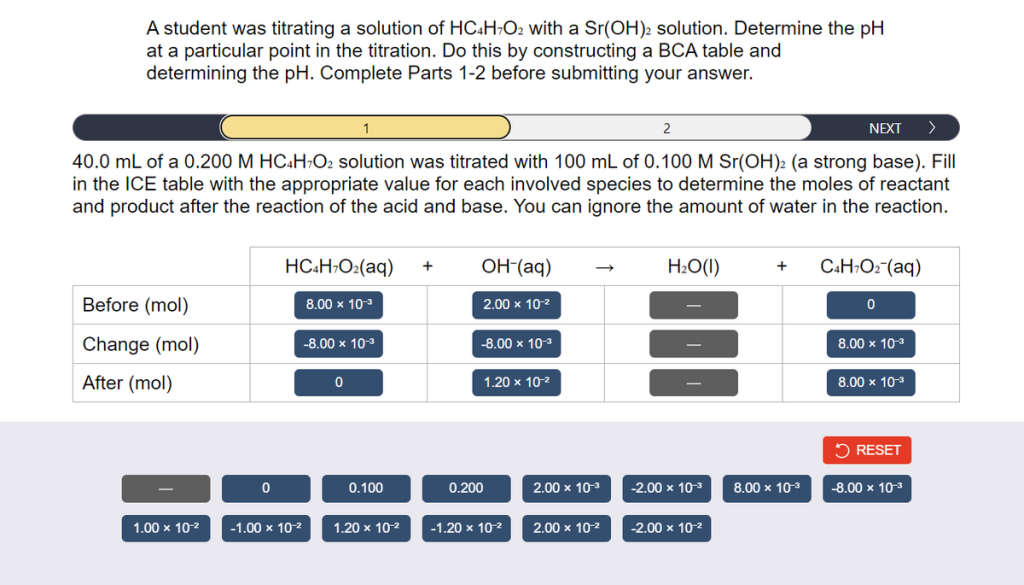
A student was titrating a solution of HC4H7O2 with a Sr(OH)2 solution. Determine the pH at a particular point in the titration. Do this by constructing a BCA table and determining the pH. Complete Parts 1-2 before submitting your answer. 1 2 NEXT > 40.0 mL of a 0.200 M HC4H7O2 solution was titrated with 100 mL of 0.100 M Sr(OH)2 (a strong base). Fill in the ICE table with the appropriate value for each involved species to determine the moles of reactant and product after the reaction of the acid and base. You can ignore the amount of water in the reaction. Before (mol) Change (mol) After (mol) 1.00 x 10-² 0 HC4H7O2(aq) + -1.00 x 10-² 8.00 x 10-³ -8.00 x 10-³ 0 0.100 1.20 x 10-² OH-(aq) 2.00 x 10-² -8.00 × 10-³ 0.200 1.20 x 10-² -1.20 x 10-² 2.00 x 10-³ 2.00 x 10-² H2O(l) -2.00 x 10-³ -2.00 x 10-² + C4H7O2 (aq) 8.00 x 10-³ 0 8.00 x 10-³ 8.00 × 10-³ RESET -8.00 x 10-³
The Correct Answer and Explanation is :
In this titration scenario, we are determining the pH after a reaction between a weak acid, HC4H7O2 (called sodium hydrogen tartrate), and a strong base, Sr(OH)2 (strontium hydroxide). We begin by constructing the ICE (Initial, Change, Equilibrium) table to track the progress of the reaction and determine the concentration of the resulting species.
Step-by-step Calculation and ICE Table
Balanced Equation:
The balanced reaction between HC4H7O2 and Sr(OH)2 is as follows:
[ \text{HC}_4\text{H}_7\text{O}_2 (aq) + \text{Sr(OH)}_2 (aq) \rightarrow \text{C}_4\text{H}_7\text{O}_2^- (aq) + \text{H}_2O (l) + \text{Sr}^{2+} (aq) ]
Given Data:
- Volume of HC4H7O2 = 40.0 mL = 0.0400 L
- Molarity of HC4H7O2 = 0.200 M
- Volume of Sr(OH)2 = 100.0 mL = 0.100 L
- Molarity of Sr(OH)2 = 0.100 M
Step 1: Calculate moles of reactants.
- Moles of HC4H7O2 = 0.200 M × 0.0400 L = 0.00800 mol
- Moles of Sr(OH)2 = 0.100 M × 0.100 L = 0.0100 mol
Step 2: Construct the ICE Table
| Species | Before (mol) | Change (mol) | After (mol) |
|---|---|---|---|
| HC4H7O2 (aq) | 0.00800 | -0.00800 | 0 |
| Sr(OH)2 (aq) | 0.0100 | -0.00800 | 0.00200 |
| OH⁻ (aq) | 0 | +0.0160 | 0.0160 |
| C4H7O2⁻ (aq) | 0 | +0.00800 | 0.00800 |
| H2O (l) | – | +0.00800 | – |
Step 3: Determine the pH
After the reaction, the limiting reagent was HC4H7O2, and the leftover strong base, Sr(OH)2, contributes OH⁻ ions to the solution. The concentration of OH⁻ is calculated from the remaining moles of OH⁻.
- Remaining OH⁻: 0.0160 mol in a total volume of 140.0 mL (40.0 mL HC4H7O2 + 100.0 mL Sr(OH)2).
- Concentration of OH⁻: ( \frac{0.0160 \, \text{mol}}{0.140 \, \text{L}} = 0.114 \, \text{M} )
Now, calculate the pOH:
[
\text{pOH} = -\log [OH^-] = -\log(0.114) = 0.943
]
Finally, calculate the pH using the relationship ( \text{pH} + \text{pOH} = 14 ):
[
\text{pH} = 14 – 0.943 = 13.06
]
Conclusion:
The pH of the solution after the titration is approximately 13.06. This result indicates that the solution is strongly basic, which is expected because the strong base (Sr(OH)2) was in excess after neutralizing the weak acid (HC4H7O2).