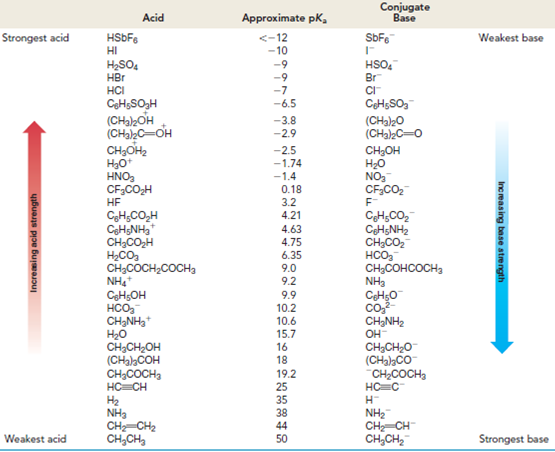
As noted in Table 3.1, the pKa of acetone, CH3COCH3, is 19.2.
(a) Draw the bond-line formula of acetone and of any other contributing resonance form.
(b) Predict and draw the structure of the conjugate base of acetone and of any other contributing resonance form.
(c) Write an equation for a reaction that could be used to synthesize CH3COCH2D.
The Correct Answer and Explanation is :
(a) Bond-Line Formula of Acetone
The bond-line formula of acetone (CH₃COCH₃) can be written as follows:
CH₃-C-CH₃
||
OThis is a simple structure where two methyl groups (CH₃) are attached to a central carbonyl group (C=O).
(b) Conjugate Base of Acetone
The conjugate base of acetone is formed when the hydrogen ion (H⁺) is removed from one of the methyl groups. In this case, acetone can lose a proton from one of the CH₃ groups, resulting in the formation of a negatively charged oxygen, forming the conjugate base. The resonance forms show the delocalization of the negative charge onto the oxygen atom.
CH₂-C-CH₃
||
O⁻This is the primary structure of the conjugate base. The negative charge on the oxygen is stabilized due to resonance with the adjacent carbonyl group, although there’s no formal resonance involving the alkyl groups (since the oxygen is more electronegative). The negative charge is localized on the oxygen in the conjugate base.
(c) Reaction to Synthesize CH₃COCH₂D
To synthesize CH₃COCH₂D (acetone-d), where the hydrogen atom on the CH₂ group is replaced with deuterium (D), we need to introduce deuterium into the reaction. This can be achieved through a reaction with deuterium oxide (D₂O) in the presence of a base.
Here’s the reaction equation for the synthesis:
CH₃COCH₃ + D₂O → CH₃COCH₂D + H₂O
Explanation:
- Acetone (CH₃COCH₃) reacts with deuterium oxide (D₂O), where D₂O provides deuterium atoms (D).
- The proton from the methylene group (CH₂) in acetone is exchanged with deuterium from D₂O, resulting in the formation of acetone-d (CH₃COCH₂D).
- In this exchange, the hydrogen atom from the methylene group is replaced by deuterium, creating a deuterated compound.
- The by-product is water (H₂O), where the oxygen in the water molecule comes from D₂O, and the hydrogen in the water is the proton that was displaced from acetone.
This reaction works because of the tendency of deuterium to exchange with hydrogen atoms in the presence of a base, facilitating the formation of the deuterated product.