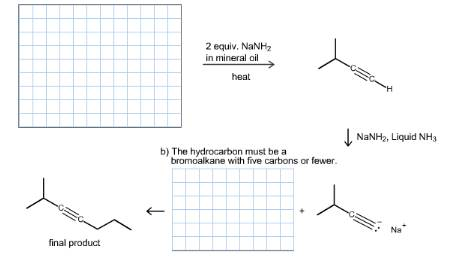
Draw the structures of the starting materials needed to make 2-methylhept-3-yne in the spaces provided. The starting materials may be any bromoalkane having five carbons or fewer.
a) The starting hydrocarbon must be a bromoalkane with five carbons or fewer.
b) The hydrocarbon must be a bromoalkane with five carbons or fewer.
The Correct Answer and Explanation is :
To prepare 2-methylhept-3-yne, you will need to use two key reactions, involving a bromoalkane with five carbons or fewer as starting materials.
Step-by-Step Explanation:
- Starting Material Selection: The first step is choosing a bromoalkane with five carbons or fewer. Based on the structure of 2-methylhept-3-yne (which has a triple bond at the 3-position and a methyl group at the 2-position), we need to construct a chain of seven carbons with these substituents. The ideal starting bromoalkane is 1-bromo-3-methylbutane (C5H11Br). This compound is a bromoalkane with five carbon atoms, which fits the requirement.
- Elimination Reaction: The synthesis proceeds by an elimination reaction. In the presence of a strong base (like potassium hydroxide, KOH), 1-bromo-3-methylbutane undergoes a dehydrobromination reaction. The base abstracts a hydrogen atom from the β-carbon (the second carbon from the bromine), leading to the formation of a double bond between the second and third carbons.
- Formation of 2-methylpent-2-ene: After this elimination, the product formed is 2-methylpent-2-ene, which has a double bond between carbons 2 and 3 and a methyl group attached to carbon 2.
- Alkyne Formation: Next, an additional halogenation and elimination reaction are required to convert the alkene into an alkyne. React the 2-methylpent-2-ene with a halogen, like bromine, in the presence of a strong base (for example, sodium amide, NaNH2). This will result in a bromine elimination reaction, where a second hydrogen atom is abstracted from the β-carbon, resulting in a triple bond between carbons 2 and 3.
- Product Formation: The final product is 2-methylhept-3-yne, which contains a triple bond at the 3-position and a methyl group at the 2-position.
Structure of 1-bromo-3-methylbutane:
- The structure of 1-bromo-3-methylbutane is:
CH3-CH2-CH(Br)-CH3
In this structure, the bromo group is on carbon 1, with a methyl group on carbon 3.
Conclusion:
Thus, 1-bromo-3-methylbutane is the starting material needed to synthesize 2-methylhept-3-yne. Through elimination reactions, it undergoes a transformation into 2-methylpent-2-ene, which then undergoes another elimination to form the desired alkyne.