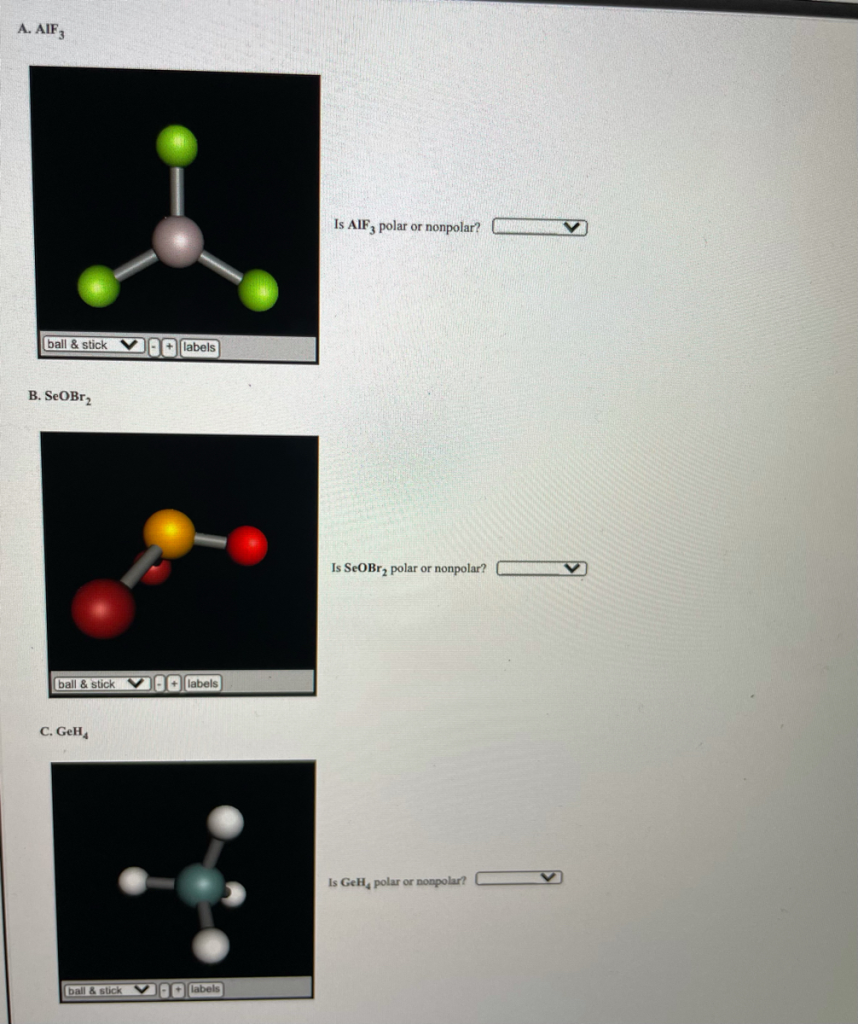
Is AlF3 polar or nonpolar?
The Correct Answer and Explanation is :
AlF₃ (Aluminum fluoride) is a nonpolar molecule.
To explain why, we need to understand the structure and bonding in AlF₃. Aluminum fluoride has a trigonal planar geometry, with the central aluminum atom bonded to three fluorine atoms arranged symmetrically around it. This symmetry leads to a cancellation of the individual dipoles created by the bonds between aluminum and fluorine. Each Al-F bond is polar due to the difference in electronegativity between aluminum and fluorine (fluorine is more electronegative), which would normally create a dipole moment. However, because the molecule has perfect symmetry, the three bond dipoles cancel each other out, resulting in no overall dipole moment for the entire molecule.
In general, the key factor for determining whether a molecule is polar or nonpolar is its symmetry. If the molecule is symmetrical, even if it contains polar bonds, the dipoles may cancel out, making the molecule nonpolar. In contrast, if the molecule is asymmetrical, the dipoles do not cancel and the molecule is polar.
It’s important to note that while AlF₃ is nonpolar in its molecular form, when it is dissolved in water or in the solid state, its ionic character plays a significant role. In its solid form, AlF₃ forms a lattice structure of Al³⁺ and F⁻ ions, which is very polar. However, when considering the molecular geometry in a vacuum or in isolation, AlF₃ is nonpolar due to the symmetrical arrangement of the atoms.