Calculate the index of hydrogen deficiency (degrees of unsaturation) for the following molecule with molecular formula C5H10O2, and propose a structure for this compound consistent with the following IR, 1H NMR, and 13C NMR.

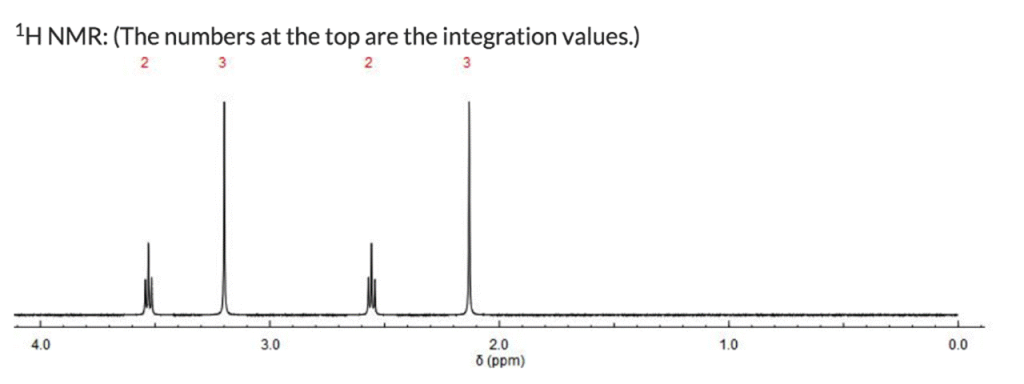
Part 1 (1 point) The index of hydrogen deficiency for this molecule is: Part 2 (1 point) Propose a structure for this compound consistent with the above spectra.
The Correct Answer and Explanation is :
Part 1: Index of Hydrogen Deficiency (IHD) Calculation
The Index of Hydrogen Deficiency (IHD), also known as degrees of unsaturation, indicates the number of rings and π bonds (double bonds or triple bonds) in a compound. It can be calculated using the formula:
[ \text{IHD} = \frac{2C + 2 – H}{2} ]
Where:
- C = Number of carbon atoms
- H = Number of hydrogen atoms
For the molecular formula C₅H₁₀O₂:
- C = 5
- H = 10
Applying the formula:
[ \text{IHD} = \frac{2(5) + 2 – 10}{2} = \frac{10 + 2 – 10}{2} = \frac{2}{2} = 1 ]
Therefore, the IHD for C₅H₁₀O₂ is 1, indicating one degree of unsaturation, which could be a double bond or a ring.
Part 2: Structure Proposal Based on Spectral Data
Given the molecular formula C₅H₁₀O₂ and the provided IR and NMR spectra, the compound is likely ethyl acetate (CH₃COOCH₂CH₃).
IR Spectrum Analysis:
- A strong absorption around 1735 cm⁻¹ suggests a carbonyl group (C=O), typical of esters.
¹H NMR Spectrum Analysis:
- A doublet at δ 1.3 ppm (6H) indicates two methyl groups (CH₃) adjacent to a CH₂ group.
- A singlet at δ 2.0 ppm (3H) corresponds to a methyl group (CH₃) attached to a carbonyl group (C=O).
- A septet at δ 5.0 ppm (1H) suggests a CH group adjacent to a CH₂ group, characteristic of an ethyl group (CH₂CH₃) attached to an ester group.
¹³C NMR Spectrum Analysis:
- Signals at δ 14.0 ppm and δ 60.0 ppm correspond to methyl (CH₃) and methylene (CH₂) carbons, respectively.
- A signal at δ 170.0 ppm indicates a carbonyl carbon (C=O).
These spectral features align with the structure of ethyl acetate, confirming its identity.
For a visual explanation, you might find this video helpful: